Loss of Dnmt3b in Chondrocytes Leads to Delayed Endochondral Ossification and Fracture Repair
- PMID: 29024060
- PMCID: PMC5809267
- DOI: 10.1002/jbmr.3305
Loss of Dnmt3b in Chondrocytes Leads to Delayed Endochondral Ossification and Fracture Repair
Abstract
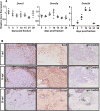
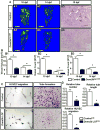
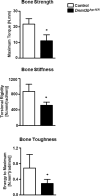
Despite advanced understanding of signaling mediated by local and systemic factors, the role of epigenetic factors in the regulation of bone regeneration remains vague. The DNA methyltransferases (Dnmts) Dnmt3a and Dnmt3b have tissue specific expression patterns and create unique methylation signatures to regulate gene expression. Using a stabilized murine tibia fracture model we find that Dnmt3b is induced early in fracture healing, peaks at 10 days post fracture (dpf), and declines to nearly undetectable levels by 28 dpf. Dnmt3b expression was cell-specific and stage-specific. High levels were observed in chondrogenic lineage cells within the fracture callus. To determine the role of Dnmt3b in fracture healing, Agc1CreERT2 ;Dnmt3bf/f (Dnmt3bAgc1ER ) mice were generated to delete Dnmt3b in chondrogenic cells. Dnmt3bAgc1ER fracture displayed chondrogenesis and chondrocyte maturation defect, and a delay in the later events of angiogenesis, ossification, and bone remodeling. Biomechanical studies demonstrated markedly reduced strength in Dnmt3bAgc1ER fractures and confirmed the delay in repair. The angiogenic response was reduced in both vessel number and volume at 10 and 14 dpf in Dnmt3bAgc1ER mice. Immunohistochemistry showed decreased CD31 expression, consistent with the reduced angiogenesis. Finally, in vitro angiogenesis assays with human umbilical vein endothelial cells (HUVECs) revealed that loss of Dnmt3b in chondrocytes significantly reduced tube formation and endothelial migration. To identify specific angiogenic factors involved in the decreased callus vascularization, a protein array was performed using conditioned media isolated from control and Dnmt3b loss-of-function chondrocytes. Several angiogenic factors, including CXCL12 and osteopontin (OPN) were reduced in chondrocytes following loss of Dnmt3b. DNA methylation analysis further identified hypomethylation in Cxcl12 promoter region. Importantly, the defects in tube formation and cell migration could be rescued by administration of CXCL12 and/or OPN. Altogether, our findings establish that Dnmt3b positively regulates chondrocyte maturation process, and its genetic ablation leads to delayed angiogenesis and fracture repair. © 2017 American Society for Bone and Mineral Research.
Keywords: ANGIOGENESIS; CHONDROCYTE; DNMT3B; FRACTURE.
© 2017 American Society for Bone and Mineral Research.
Conflict of interest statement
All authors state that they have no conflicts of interest.
Figures



References
-
- Vortkamp A, Pathi S, Peretti GM, Caruso EM, Zaleske DJ, Tabin CJ. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech Dev. 1998;71(1–2):65–76. - PubMed
-
- Ferguson C, Alpern E, Miclau T, Helms JA. Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev. 1999;87(1–2):57–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

