Functional interleukin-6 receptor-α is located in tanycytes at the base of the third ventricle
- PMID: 29024103
- PMCID: PMC5852644
- DOI: 10.1111/jne.12546
Functional interleukin-6 receptor-α is located in tanycytes at the base of the third ventricle
Abstract
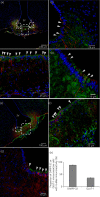
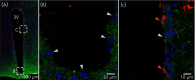
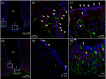
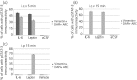
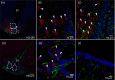
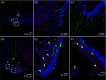
Interleukin (IL)-6- /- mice develop mature onset obesity, whereas i.c.v. injection of IL-6 decreases obesity in rodents. Moreover, levels of IL-6 in cerebrospinal fluid (CSF) were reported to be inversely correlated with obesity in humans. Tanycytes lining the base of the third ventricle (3V) in the hypothalamus have recently been reported to be of importance for metabolism. In the present study, we investigated whether tanycytes could respond to IL-6 in the CSF. With immunohistochemistry using a well characterised antibody directed against the ligand binding receptor for IL-6, IL-6 receptor α (IL-6Rα), it was found that tanycytes, identified by the two markers, vimentin and dopamine- and cAMP-regulated phosphoprotein of 32 kDa, contained IL-6Rα. There were fewer IL-6Rα on another type of ventricle-lining cells, ependymal cells, as identified by the marker glucose transporter-1. To demonstrate that the immunoreactive IL-6Rα were responsive to IL-6, we injected IL-6 i.c.v. This treatment increased immunoreactive phosphorylated signal transducer and activator of transcription-3 (pSTAT3) in tanycytes after 5 minutes and in cells in the medial part of the arcuate nucleus after 5 and 15 minutes. Intracerebroventricular injection of leptin exerted similar effects. As expected, i.p. injection of leptin also induced pSTAT3 staining in the hypothalamus, whereas i.p. IL-6 injection had little effect on this parameter. Intracerebroventricular or i.p. injection of vehicle only had no effect on pSTAT3-immunoreactivity. In summary, there are functional IL-6Rα on tanycytes at the bottom of the 3V, in agreement with the possibility that ventricular administration of IL-6 decreases obesity in mice via an effect on this cell type.
Keywords: IL-6; IL-6Rα; hypothalamus; tanycytes; third ventricle.
© 2017 The Authors. Journal of Neuroendocrinology published by John Wiley & Sons Ltd. on behalf of British Society for Neuroendocrinology.
Figures
Similar articles
-
Hepatic leptin receptor expression can partially compensate for IL-6Rα deficiency in DEN-induced hepatocellular carcinoma.Mol Metab. 2018 Nov;17:122-133. doi: 10.1016/j.molmet.2018.08.010. Epub 2018 Sep 5. Mol Metab. 2018. PMID: 30224299 Free PMC article.
-
α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors.Nat Commun. 2013;4:2049. doi: 10.1038/ncomms3049. Nat Commun. 2013. PMID: 23804023
-
Activation of the hypothalamic arcuate nucleus predicts the anorectic actions of ciliary neurotrophic factor and leptin in intact and gold thioglucose-lesioned mice.J Neuroendocrinol. 2003 Jul;15(7):649-60. doi: 10.1046/j.1365-2826.2003.01043.x. J Neuroendocrinol. 2003. PMID: 12787049
-
Targeting Tanycytes: Balance between Efficiency and Specificity.Neuroendocrinology. 2020;110(7-8):574-581. doi: 10.1159/000505549. Epub 2020 Jan 2. Neuroendocrinology. 2020. PMID: 31986518 Review.
-
Tanycytes in the infundibular nucleus and median eminence and their role in the blood-brain barrier.Handb Clin Neurol. 2021;180:253-273. doi: 10.1016/B978-0-12-820107-7.00016-1. Handb Clin Neurol. 2021. PMID: 34225934 Review.
Cited by
-
Interleukin-6 in the central amygdala is bioactive and co-localised with glucagon-like peptide-1 receptor.J Neuroendocrinol. 2019 Jun;31(6):e12722. doi: 10.1111/jne.12722. Epub 2019 May 23. J Neuroendocrinol. 2019. PMID: 31033078 Free PMC article.
-
Regulation of Energy Expenditure and Brown/Beige Thermogenic Activity by Interleukins: New Roles for Old Actors.Int J Mol Sci. 2018 Aug 29;19(9):2569. doi: 10.3390/ijms19092569. Int J Mol Sci. 2018. PMID: 30158466 Free PMC article. Review.
-
Parabrachial Interleukin-6 Reduces Body Weight and Food Intake and Increases Thermogenesis to Regulate Energy Metabolism.Cell Rep. 2019 Mar 12;26(11):3011-3026.e5. doi: 10.1016/j.celrep.2019.02.044. Cell Rep. 2019. PMID: 30865890 Free PMC article.
-
Keratinocyte-Derived Cytokine in the Hippocampus Disrupts Extinction of Conditioned Fear Memory in Tumor-Bearing Mice.Mol Neurobiol. 2024 Sep;61(9):6454-6468. doi: 10.1007/s12035-024-03992-1. Epub 2024 Feb 3. Mol Neurobiol. 2024. PMID: 38308664
-
Nacre extract from pearl oyster attenuates amyloid beta-induced memory impairment.J Nat Med. 2022 Mar;76(2):419-434. doi: 10.1007/s11418-021-01598-8. Epub 2022 Jan 19. J Nat Med. 2022. PMID: 35044595
References
-
- Hodge D, Hurt E, Farrar W. The role of IL‐6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502‐2512. - PubMed
-
- Ishihara K, Hirano T. IL‐6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13:357‐368. - PubMed
-
- Mauer J, Denson J, Brüning J. Versatile functions for IL‐6 in metabolism and cancer. Trends Immunol. 2015;36:92‐101. - PubMed
-
- Pedersen B, Febbraio M. Muscle as an endocrine organ: focus on muscle‐derived interleukin‐6. Physiol Rev. 2008;88:1379‐1406. - PubMed
-
- Kamimura D, Ishihara K, Hirano T. IL‐6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1‐38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

