Not in your usual Top 10: protists that infect plants and algae
- PMID: 29024322
- PMCID: PMC5772912
- DOI: 10.1111/mpp.12580
Not in your usual Top 10: protists that infect plants and algae
Abstract
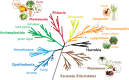
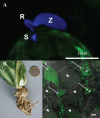
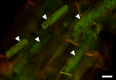
Fungi, nematodes and oomycetes belong to the most prominent eukaryotic plant pathogenic organisms. Unicellular organisms from other eukaryotic lineages, commonly addressed as protists, also infect plants. This review provides an introduction to plant pathogenic protists, including algae infecting oomycetes, and their current state of research.
Keywords: algae; phytomonas; phytomyxae; plant pathogens; plasmodiophorids; protist; stramenopiles.
© 2017 THE AUTHORS. MOLECULAR PLANT PATHOLOGY PUBLISHED BY BRITISH SOCIETY FOR PLANT PATHOLOGY AND JOHN WILEY & SONS LTD.
Figures





References
-
- Adl, S.M. , Simpson, A.G.B. , Lane, C.E. , Lukes, J. , Bass, D. , Bowser, S.S. , Brown, M.W. , Burki, F. , Dunthorn, M. , Hampl, V. , Heiss, A. , Hoppenrath, M. , Lara, E. , Le Gall, L. , Lynn, D.H. , McManus, H. , Mitchell, E.A. , Mozley‐Stanridge, S.E. , Parfrey, L.W. , Pawlowski, J. , Rueckert, S. , Shadwick, L. , Schoch, C.L. , Smirnov, A. and Spiegel, F.W. (2012) The revised classification of eukaryotes. J. Eukaryot. Microbiol. 59, 429–493. - PMC - PubMed
-
- Agarwal, A. , Kaul, V. , Faggian, R. , Rookes, J.E. , Ludwig‐Muller, J. and Cahill, D.M. (2011) Analysis of global host gene expression during the primary phase of the Arabidopsis thaliana–Plasmodiophora brassicae interaction. Funct. Plant Biol. 38, 462–478. - PubMed
-
- Aist, J.R. and Williams, P.H. (1971) The cytology and kinetics of cabbage root hair penetration by Plasmodiophora brassicae . Can. J. Bot. 49, 2023–2034.
-
- Amano, H. , Suginaga, R. , Arashima, K. and Noda, H. (1995) Immunological detection of the fungal parasite, Pythium sp. – the causative organism of red rot disease in Porphyra‐yezoensis . J. Appl. Phycol. 7, 53–58.
-
- Amon, J.P. (1978) Thraustochytrids and labyrinthulids of terrestrial, aquatic and hypersaline environments of the Great Salt Lake, USA. Mycologia, 70, 1299–1301.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

