Integrin β-3 is required for the attachment, retention and therapeutic benefits of human cardiospheres in myocardial infarction
- PMID: 29024385
- PMCID: PMC5742734
- DOI: 10.1111/jcmm.13325
Integrin β-3 is required for the attachment, retention and therapeutic benefits of human cardiospheres in myocardial infarction
Abstract
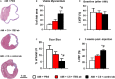
Cardiovascular diseases remain the leading causes of death worldwide. Stem cell therapy offers a promising option to regenerate injured myocardium. Among the various types of stem cells, cardiosphere cells represent a mixture of intrinsic heart stem cells and supporting cells. The safety and efficacy of cardiosphere cells have been demonstrated in recent clinical trials. Cell-matrix interaction plays an important role in mediating the engraftment of injected stem cells. Here, we studied the role of integrin β-3 in cardiosphere-mediated cell therapy in a mouse model of myocardial infarction. Our results indicated that inhibiting integrin β-3 reduced attachment, retention and therapeutic benefits of human cardiospheres in mice with acute myocardial infarction. This suggests integrin β-3 plays an important role in cardiosphere-mediated heart regeneration.
Keywords: cardiac stem cells; cardiospheres; integrin; myocardial infarction.
© 2017 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures




Similar articles
-
Dose-dependent functional benefit of human cardiosphere transplantation in mice with acute myocardial infarction.J Cell Mol Med. 2012 Sep;16(9):2112-6. doi: 10.1111/j.1582-4934.2011.01512.x. J Cell Mol Med. 2012. PMID: 22225626 Free PMC article.
-
Generation of Induced Cardiospheres via Reprogramming of Skin Fibroblasts for Myocardial Regeneration.Stem Cells. 2016 Nov;34(11):2693-2706. doi: 10.1002/stem.2438. Epub 2016 Jul 7. Stem Cells. 2016. PMID: 27333945
-
Pericardial application as a new route for implanting stem-cell cardiospheres to treat myocardial infarction.J Physiol. 2018 Jun;596(11):2037-2054. doi: 10.1113/JP275548. Epub 2018 May 7. J Physiol. 2018. PMID: 29736937 Free PMC article.
-
Cardiospheres and cardiosphere-derived cells as therapeutic agents following myocardial infarction.Expert Rev Cardiovasc Ther. 2012 Sep;10(9):1185-94. doi: 10.1586/erc.12.102. Expert Rev Cardiovasc Ther. 2012. PMID: 23098154 Review.
-
[Perspectives of cell therapy for myocardial infarction and heart failure based on cardiosphere cells].Ter Arkh. 2020 May 19;92(4):111-120. doi: 10.26442/00403660.2020.04.000634. Ter Arkh. 2020. PMID: 32598708 Review. Russian.
Cited by
-
The Future of Direct Cardiac Reprogramming: Any GMT Cocktail Variety?Int J Mol Sci. 2020 Oct 26;21(21):7950. doi: 10.3390/ijms21217950. Int J Mol Sci. 2020. PMID: 33114756 Free PMC article. Review.
-
Key Roles of RGD-Recognizing Integrins During Cardiac Development, on Cardiac Cells, and After Myocardial Infarction.J Cardiovasc Transl Res. 2022 Feb;15(1):179-203. doi: 10.1007/s12265-021-10154-4. Epub 2021 Aug 3. J Cardiovasc Transl Res. 2022. PMID: 34342855 Review.
-
Possible Treatment of Myocardial Infarct Based on Tissue Engineering Using a Cellularized Solid Collagen Scaffold Functionalized with Arg-Glyc-Asp (RGD) Peptide.Int J Mol Sci. 2021 Nov 22;22(22):12563. doi: 10.3390/ijms222212563. Int J Mol Sci. 2021. PMID: 34830447 Free PMC article. Review.
-
PEX3 promotes regenerative repair after myocardial injury in mice through facilitating plasma membrane localization of ITGB3.Commun Biol. 2024 Jul 1;7(1):795. doi: 10.1038/s42003-024-06483-0. Commun Biol. 2024. PMID: 38951640 Free PMC article.
-
Exploration and validation of hub genes and pathways in the progression of hypoplastic left heart syndrome via weighted gene co-expression network analysis.BMC Cardiovasc Disord. 2021 Jun 15;21(1):300. doi: 10.1186/s12872-021-02108-0. BMC Cardiovasc Disord. 2021. PMID: 34130651 Free PMC article.
References
-
- Smith RR, Barile L, Cho HC, et al Regenerative potential of cardiosphere‐derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007; 115: 896–908. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

