A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (EpiFix® ) allograft for the treatment of venous leg ulcers
- PMID: 29024419
- PMCID: PMC7949978
- DOI: 10.1111/iwj.12843
A multicentre randomised controlled trial evaluating the efficacy of dehydrated human amnion/chorion membrane (EpiFix® ) allograft for the treatment of venous leg ulcers
Abstract
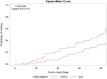
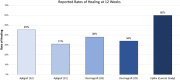
A randomised, controlled, multicentre clinical trial was conducted to evaluate the efficacy of dehydrated human amnion/chorion membrane (EpiFix) allograft as an adjunct to multilayer compression therapy for the treatment of non-healing full-thickness venous leg ulcers. We randomly assigned 109 subjects to receive EpiFix and multilayer compression (n = 52) or dressings and multilayer compression therapy alone (n = 57). Patients were recruited from 15 centres around the USA and were followed up for 16 weeks. The primary end point of the study was defined as time to complete ulcer healing. Participants receiving weekly application of EpiFix and compression were significantly more likely to experience complete wound healing than those receiving standard wound care and compression (60% versus 35% at 12 weeks, P = 0·0128, and 71% versus 44% at 16 weeks, P = 0·0065). A Kaplan-Meier analysis was performed to compare the time-to-healing performance with or without EpiFix, showing a significantly improved time to healing using the allograft (log-rank P = 0·0110). Cox regression analysis showed that subjects treated with EpiFix had a significantly higher probability of complete healing within 12 weeks (HR: 2·26, 95% confidence interval 1·25-4·10, P = 0·01) versus without EpiFix. These results confirm the advantage of EpiFix allograft as an adjunct to multilayer compression therapy for the treatment of non-healing, full-thickness venous leg ulcers.
Keywords: Amniotic membrane allografts; Chronic wounds; Dehydrated human amnion/chorion membrane allograft; Venous leg ulcers.
© 2017 The Authors. International Wound Journal published by Medicalhelplines.com Inc and John Wiley & Sons Ltd.
Figures
References
-
- Lazarus G, Valle MF, Malas M, Qazi U, Maruthur NM, Doggett D, Fawole OA, Bass EB, Zenilman J. Chronic venous leg ulcer treatment: future research needs. Wound Repair Regen 2014;22:34–42. - PubMed
-
- Ballard JL, Bergan JJ. Chronic venous insufficiency. Diagnosis and treatment. London: Springer‐Verlag, 2000.
-
- McDaniel HB, Marston WA, Farber MA, Mendes RR, Owens LV, Young ML, Daniel PF, Keagy BA. Recurrence of chronic venous ulcers on the basis of clinical, etiologic, anatomic, and pathophysiologic criteria and air plethysmography. J Vasc Surg 2002;35(4):723–8. - PubMed
-
- Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. J Med Econ 2014;17(5):347–56. - PubMed
-
- Bergan JJ, Schmid‐Schönbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med 2006;355(5):488–98. Review. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical