Bioengineered lungs generated from human iPSCs-derived epithelial cells on native extracellular matrix
- PMID: 29024475
- PMCID: PMC5991621
- DOI: 10.1002/term.2589
Bioengineered lungs generated from human iPSCs-derived epithelial cells on native extracellular matrix
Abstract
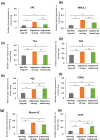
The development of an alternative source for donor lungs would change the paradigm of lung transplantation. Recent studies have demonstrated the potential feasibility of using decellularized lungs as scaffolds for lung tissue regeneration and subsequent implantation. However, finding a reliable cell source and the ability to scale up for recellularization of the lung scaffold still remain significant challenges. To explore the possibility of regeneration of human lung tissue from stem cells in vitro, populations of lung progenitor cells were generated from human iPSCs. To explore the feasibility of producing engineered lungs from stem cells, we repopulated decellularized human lung and rat lungs with iPSC-derived epithelial progenitor cells. The iPSCs-derived epithelial progenitor cells lined the decellularized human lung and expressed most of the epithelial markers when were cultured in a lung bioreactor system. In decellularized rat lungs, these human-derived cells attach and proliferate in a manner similar to what was observed in the decellularized human lung. Our results suggest that repopulation of lung matrix with iPSC-derived lung epithelial cells may be a viable strategy for human lung regeneration and represents an important early step toward translation of this technology.
Keywords: decellularized lung; epithelial cells; induced pluripotent stem cell (iPSC); lung implant; tissue regeneration bioreactor.
Copyright © 2017 John Wiley & Sons, Ltd.
Figures





References
-
- Ali NN, Edgar AJ, Samadikuchaksaraei A, Timson CM, Romanska HM, Polak JM, Bishop AE. Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Engineering. 2002;8(4):541–550. - PubMed
-
- Badylak SF, Weiss DJ, Caplan A, Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379(9819):943–952. - PubMed
-
- Balestrini JL, Gard AL, Liu A, Leiby KL, Schwan J, Kunkemoeller B, Niklason LE. Production of decellularized porcine lung scaffolds for use in tissue engineering. Integrative Biology (Camb) 2015;7(12):1598–1610. https://doi.org/10.1039/c5ib00063g. - DOI - PMC - PubMed
-
- Beers MF, Moodley Y group RSCW. When is an alveolar type 2 cell an alveolar type 2 cell? A conundrum for lung stem cell biology and regenerative medicine. American Journal of Respiratory Cell and Molecular Biology. 2017 https://doi.org/10.1165/rcmb.2016-0426PS. [Epub ahead of print] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

