Placenta-Derived Adherent Stromal Cells Improve Diabetes Mellitus-Associated Left Ventricular Diastolic Performance
- PMID: 29024485
- PMCID: PMC5702519
- DOI: 10.1002/sctm.17-0130
Placenta-Derived Adherent Stromal Cells Improve Diabetes Mellitus-Associated Left Ventricular Diastolic Performance
Abstract
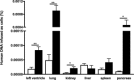
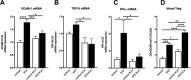
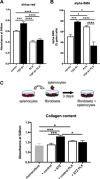
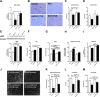
Left ventricular (LV) diastolic dysfunction is among others attributed to cardiomyocyte stiffness. Mesenchymal stromal cells (MSC) have cardiac-protective properties. We explored whether intravenous (i.v.) application of PLacenta-eXpanded (PLX) MSC-like cells (PLX) improves LV diastolic relaxation in streptozotocin (STZ)-induced diabetic mice and investigated underlying mechanisms. Diabetes mellitus was induced by STZ application (50 mg/kg body weight) during five subsequent days. One week after the first STZ injection, PLX or saline were i.v. applied. Two weeks later, mice were hemodynamically characterized and sacrificed. At this early stage of diabetic cardiomyopathy with low-grade inflammation and no cardiac fibrosis, PLX reduced LV vascular cell adhesion molecule-1, transforming growth factor-β1, and interferon-γ mRNA expression, induced the percentage of circulating regulatory T cells, and decreased the splenic pro-fibrotic potential in STZ mice. STZ + PLX mice exhibited higher LV vascular endothelial growth factor mRNA expression and arteriole density versus STZ mice. In vitro, hyperglycemic PLX conditioned medium restored the hyperglycemia-impaired tube formation and adhesion capacity of human umbelical vein endothelial cells (HUVEC) via increasing nitric oxide (NO) bioavailability. PLX further induced the diabetes-downregulated activity of the NO downstream protein kinase G, as well as of protein kinase A, in STZ mice, which was associated with a raise in phosphorylation of the titin isoforms N2BA and N2B. Concomitantly, the passive force was lower in single isolated cardiomyocytes from STZ + PLX versus from STZ mice, which led to an improvement of LV diastolic relaxation. We conclude that i.v. PLX injection improves diabetes mellitus-associated diastolic performance via decreasing cardiomyocyte stiffness. Stem Cells Translational Medicine 2017;6:2135-2145.
Keywords: Diabetes mellitus; Diastole; Passive force; Placenta-expanded cell; Titin.
© 2017 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures

References
-
- Van Linthout S, Spillmann F, Riad A et al. Human apolipoprotein A‐I gene transfer reduces the development of experimental diabetic cardiomyopathy. Circulation 2008;117:1563–1573. - PubMed
-
- Tschope C, Walther T, Königer J et al. Prevention of cardiac fibrosis and left ventricular dysfunction in diabetic cardiomyopathy in rats by transgenic expression of the human tissue kallikrein gene. FASEB J 2004;18:828–835. - PubMed
-
- Farhangkhoee H, Khan ZA, Kaur H et al. Vascular endothelial dysfunction in diabetic cardiomyopathy: Pathogenesis and potential treatment targets. Pharmacol Ther 2006;111:384–399. - PubMed
-
- Kruger M, Babicz K, von Frieling‐Salewsky M et al. Insulin signaling regulates cardiac titin properties in heart development and diabetic cardiomyopathy. J Mol Cell Cardiol 2010;48:910–916. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

