Adrenomedullin improves fertility and promotes pinopodes and cell junctions in the peri-implantation endometrium
- PMID: 29025060
- PMCID: PMC6248476
- DOI: 10.1093/biolre/iox101
Adrenomedullin improves fertility and promotes pinopodes and cell junctions in the peri-implantation endometrium
Abstract
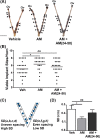
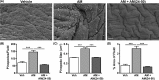
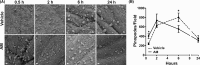
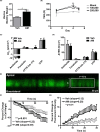
Implantation is a complex event demanding contributions from both embryo and endometrium. Despite advances in assisted reproduction, endometrial receptivity defects persist as a barrier to successful implantation in women with infertility. We previously demonstrated that maternal haploinsufficiency for the endocrine peptide adrenomedullin (AM) in mice confers a subfertility phenotype characterized by defective uterine receptivity and sparse epithelial pinopode coverage. The strong link between AM and implantation suggested the compelling hypothesis that administration of AM prior to implantation may improve fertility, protect against pregnancy complications, and ultimately lead to better maternal and fetal outcomes. Here, we demonstrate that intrauterine delivery of AM prior to blastocyst transfer improves the embryo implantation rate and spacing within the uterus. We then use genetic decrease-of-function and pharmacologic gain-of-function mouse models to identify potential mechanisms by which AM confers enhanced implantation success. In epithelium, we find that AM accelerates the kinetics of pinopode formation and water transport and that, in stroma, AM promotes connexin 43 expression, gap junction communication, and barrier integrity of the primary decidual zone. Ultimately, our findings advance our understanding of the contributions of AM to uterine receptivity and suggest potential broad use for AM as therapy to encourage healthy embryo implantation, for example, in combination with in vitro fertilization.
Keywords: assisted reproductive technology; decidua; endometrium; fertility; implantation; in vitro fertilization (IVF); mechanisms of hormone action; pregnancy; uterus.
© The Authors 2017. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 2006; 7:185–199. - PubMed
-
- Scott RT Jr, Ferry K, Su J, Tao X, Scott K, Treff NR. Comprehensive chromosome screening is highly predictive of the reproductive potential of human embryos: a prospective, blinded, nonselection study. Fertil Steril 2012; 97:870–875. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

