Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections
- PMID: 29025194
- PMCID: PMC6485408
- DOI: 10.1002/14651858.CD007498.pub3
Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections
Abstract
Background: Acute respiratory infections (ARIs) comprise of a large and heterogeneous group of infections including bacterial, viral, and other aetiologies. In recent years, procalcitonin (PCT), a blood marker for bacterial infections, has emerged as a promising tool to improve decisions about antibiotic therapy (PCT-guided antibiotic therapy). Several randomised controlled trials (RCTs) have demonstrated the feasibility of using procalcitonin for starting and stopping antibiotics in different patient populations with ARIs and different settings ranging from primary care settings to emergency departments, hospital wards, and intensive care units. However, the effect of using procalcitonin on clinical outcomes is unclear. This is an update of a Cochrane review and individual participant data meta-analysis first published in 2012 designed to look at the safety of PCT-guided antibiotic stewardship.
Objectives: The aim of this systematic review based on individual participant data was to assess the safety and efficacy of using procalcitonin for starting or stopping antibiotics over a large range of patients with varying severity of ARIs and from different clinical settings.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE, and Embase, in February 2017, to identify suitable trials. We also searched ClinicalTrials.gov to identify ongoing trials in April 2017.
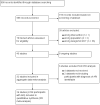
Selection criteria: We included RCTs of adult participants with ARIs who received an antibiotic treatment either based on a procalcitonin algorithm (PCT-guided antibiotic stewardship algorithm) or usual care. We excluded trials if they focused exclusively on children or used procalcitonin for a purpose other than to guide initiation and duration of antibiotic treatment.
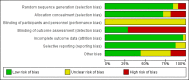
Data collection and analysis: Two teams of review authors independently evaluated the methodology and extracted data from primary studies. The primary endpoints were all-cause mortality and treatment failure at 30 days, for which definitions were harmonised among trials. Secondary endpoints were antibiotic use, antibiotic-related side effects, and length of hospital stay. We calculated odds ratios (ORs) and 95% confidence intervals (CIs) using multivariable hierarchical logistic regression adjusted for age, gender, and clinical diagnosis using a fixed-effect model. The different trials were added as random-effects into the model. We conducted sensitivity analyses stratified by clinical setting and type of ARI. We also performed an aggregate data meta-analysis.
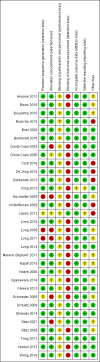
Main results: From 32 eligible RCTs including 18 new trials for this 2017 update, we obtained individual participant data from 26 trials including 6708 participants, which we included in the main individual participant data meta-analysis. We did not obtain individual participant data for four trials, and two trials did not include people with confirmed ARIs. According to GRADE, the quality of the evidence was high for the outcomes mortality and antibiotic exposure, and quality was moderate for the outcomes treatment failure and antibiotic-related side effects.Primary endpoints: there were 286 deaths in 3336 procalcitonin-guided participants (8.6%) compared to 336 in 3372 controls (10.0%), resulting in a significantly lower mortality associated with procalcitonin-guided therapy (adjusted OR 0.83, 95% CI 0.70 to 0.99, P = 0.037). We could not estimate mortality in primary care trials because only one death was reported in a control group participant. Treatment failure was not significantly lower in procalcitonin-guided participants (23.0% versus 24.9% in the control group, adjusted OR 0.90, 95% CI 0.80 to 1.01, P = 0.068). Results were similar among subgroups by clinical setting and type of respiratory infection, with no evidence for effect modification (P for interaction > 0.05). Secondary endpoints: procalcitonin guidance was associated with a 2.4-day reduction in antibiotic exposure (5.7 versus 8.1 days, 95% CI -2.71 to -2.15, P < 0.001) and lower risk of antibiotic-related side effects (16.3% versus 22.1%, adjusted OR 0.68, 95% CI 0.57 to 0.82, P < 0.001). Length of hospital stay and intensive care unit stay were similar in both groups. A sensitivity aggregate-data analysis based on all 32 eligible trials showed similar results.
Authors' conclusions: This updated meta-analysis of individual participant data from 12 countries shows that the use of procalcitonin to guide initiation and duration of antibiotic treatment results in lower risks of mortality, lower antibiotic consumption, and lower risk for antibiotic-related side effects. Results were similar for different clinical settings and types of ARIs, thus supporting the use of procalcitonin in the context of antibiotic stewardship in people with ARIs. Future high-quality research is needed to confirm the results in immunosuppressed patients and patients with non-respiratory infections.
Conflict of interest statement
Philipp Schuetz received support (paid to his employer) from Thermo Fisher, Roche Diagnostics, Abbott and bioMerieux to attend meetings and fulfil speaking engagements. These conflicts breach Cochrane's
Funding: The initial review was partly funded by unrestricted research grants from B·R·A·H·M·S/Thermo Fisher Scientific, the Gottfried and Julia Bangerter‐Rhyner‐Foundation, the Swiss Foundation for Grants in Biology and Medicine (SSMBS, PASMP3‐127684/1), and santésuisse to cover salary time related to this review. The sponsors had no role in the study design, data collection, data analysis or data interpretation, or writing of the report. No funding was received for this update.
No commercial sponsor had any involvement in the design and conduct of this review, namely collection, management, analysis, and interpretation of the data; and preparation, decision to submit, review, or approval of the manuscript.
Figures

Update of
-
Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD007498. doi: 10.1002/14651858.CD007498.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Oct 12;10:CD007498. doi: 10.1002/14651858.CD007498.pub3. PMID: 22972110 Free PMC article. Updated.
Comment in
-
Review: Procalcitonin-guided starting and stopping of antibiotics in acute respiratory infections reduces mortality.Ann Intern Med. 2018 Feb 20;168(4):JC19. doi: 10.7326/ACPJC-2018-168-4-019. Ann Intern Med. 2018. PMID: 29459956 No abstract available.
-
Is Procalcitonin-Guided Antibiotic Therapy Working in Emergency Department Outpatients?Ann Emerg Med. 2018 Aug;72(2):226-228. doi: 10.1016/j.annemergmed.2018.03.028. Ann Emerg Med. 2018. PMID: 30031515 No abstract available.
References
References to studies included in this review
Annane 2013 {published data only}
Bloos 2016 {published data only}
-
- Bloos F, Trips E, Nierhaus A, Briegel J, Heyland DK, Jaschinski U, et al. Effect of sodium selenite administration and procalcitonin‐guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial. JAMA Internal Medicine 2016;176(9):1266‐76. - PubMed
Bouadma 2010 {published data only}
-
- Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010;375(9713):463‐74. - PubMed
Branche 2015 {published data only}
Briel 2008 {published data only}
-
- Briel M, Schuetz P, Mueller B, Young J, Schild U, Nusbaumer C, et al. Procalcitonin‐guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Archives of Internal Medicine 2008;168(18):2000‐7. - PubMed
Burkhardt 2010 {published data only}
-
- Burkhardt O, Ewig S, Haagen U, Giersdorf S, Hartmann O, Wegscheider K, et al. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. European Respiratory Journal 2010;36(3):601‐7. - PubMed
Christ‐Crain 2004 {published data only}
-
- Christ‐Crain M, Jaccard‐Stolz D, Bingisser R, Gencay M, Huber P, Tamm M, et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet 2004;1363(9409):600‐7. - PubMed
Christ‐Crain 2006 {published data only}
-
- Christ‐Crain M, Stolz D, Bingisser R, Muller C, Miedinger D, Huber PR, et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. American Journal of Respiratory and Critical Care Medicine 2006;174(1):84‐93. - PubMed
Corti 2016 {published data only}
-
- Corti C, Fally M, Fabricius‐Bjerre A, Mortensen K, Jensen BN, Andreassen HF, et al. Point‐of‐care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. International Journal of Chronic Obstructructive Pulmonary Disease 2016;11:1381‐9. [DOI: 10.2147/COPD.S104051] - DOI - PMC - PubMed
De Jong 2016 {published data only}
-
- Jong E, Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open‐label trial. Lancet Infectious Diseases 2016;16(7):819‐27. - PubMed
Deliberato 2013 {published data only}
-
- Deliberato RO, Marra AR, Sanches PR, Martino MD, Ferreira CE, Pasternak J, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagnostic Microbiology and Infectious Disease 2013;76(3):266‐71. - PubMed
Ding 2013 {published data only}
Hochreiter 2009 {published data only}
Kristoffersen 2009 {published data only}
-
- Kristoffersen KB, Sogaard OS, Wejse C, Black FT, Greve T, Tarp B, et al. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission ‐ a randomized trial. Clinical Microbiology and Infection 2009;15(5):481‐7. - PubMed
Layios 2012 {published data only}
-
- Layios N, Lambermont B, Canivet JL, Morimont P, Preiser JC, Garweg C, et al. Procalcitonin usefulness for the initiation of antibiotic treatment in intensive care unit patients. Critical Care Medicine 2012;40(8):2304‐9. - PubMed
Lima 2016 {published data only}
-
- Lima SS, Nobre V, Castro Romanelli RM, Clemente WT, Silva Bittencourt HN, Melo AC, et al. Procalcitonin‐guided protocol is not useful to manage antibiotic therapy in febrile neutropenia: a randomized controlled trial. Annals of Hematology 2016;95(7):1169‐76. - PubMed
Long 2009 {published data only}
-
- Long W, Deng XQ, Tang JG, Xie J, Zhang YC, Zhang Y, et al. Procalcitonin guidance for reduction of antibiotic use in low‐risk outpatients with community acquired pneumonia. Zhonghua Nei Ke Za Zhi 2009;48(3):216‐9. - PubMed
Long 2011 {published data only}
-
- Long W, Deng X, Zhang Y, Lu G, Xie J, Tang J. Procalcitonin guidance for reduction of antibiotic use in low‐risk outpatients with community acquired pneumonia. Respirology 2011;76(1):266‐9. - PubMed
Long 2014 {published data only}
Maravić‐Stojković 2011 {published data only}
-
- Maravić‐Stojković V, Laušević‐Vuk L, Jović M, Ranković A, Borzanović M, Marinković J. Procalcitonin‐based therapeutic strategy to reduce antibiotic use in patients after cardiac surgery: a randomized controlled trial. Srpski Arhiv Celokupno Lekarstvo 2011;139(11‐12):736‐42. - PubMed
Najafi 2015 {published data only}
-
- Najafi A, Khodadadian A, Sanatkar M, Shariat Moharari R, Etezadi F, Ahmadi A, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Medica Iranica 2015;53(9):562‐7. - PubMed
Nobre 2008 {published data only}
-
- Nobre V, Harbarth S, Graf JD, Rohner P, Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. American Journal of Respiratory and Critical Care Medicine 2009;177(5):498‐505. - PubMed
Ogasawara 2014 {published data only}
-
- Ogasawara T, Umezawa H, Naito Y, Takeuchi T, Kato S, Yano T, et al. Procalcitonin‐guided antibiotic therapy in aspiration pneumonia and an assessment of the continuation of oral intake. Respiratory Investigation 2014;52(2):107‐13. - PubMed
Oliveira 2013 {published data only}
-
- Oliveira CF, Botoni FA, Oliveira CR, Silva CB, Pereira HA, Serufo JC, et al. Procalcitonin versus C‐reactive protein for guiding antibiotic therapy in sepsis: a randomized trial. Critical Care Medicine 2013;41(10):2336‐43. - PubMed
Schroeder 2009 {published data only}
-
- Schroeder S, Hochreiter M, Koehler T, Schweiger AM, Bein B, Keck FS, et al. Procalcitonin (PCT)‐guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Archives of Surgery 2009;394(2):221‐6. - PubMed
Schuetz 2009 {published data only}
-
- Schuetz P, Christ‐Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, et al. Effect of procalcitonin‐based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009;302(10):1059‐66. - PubMed
Shehabi 2014 {published data only}
-
- Shehabi Y, Sterba M, Garrett PM, Rachakonda KS, Stephens D, Harrigan P, et al. ProGUARD Study Investigators, ANZICS Clinical Trials Group. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2014;190(10):1102‐10. - PubMed
Stolz 2007 {published data only}
-
- Stolz D, Christ‐Crain M, Bingisser R, Leuppi J, Miedinger D, Muller C, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin‐guidance with standard therapy. Chest 2007;131(1):9‐19. - PubMed
Stolz 2009 {published data only}
-
- Stolz D, Smyrnios N, Eggimann P, Pargger H, Thakkar N, Siegemund M, et al. Procalcitonin for reduced antibiotic exposure in ventilator‐associated pneumonia: a randomised study. European Respiratory Journal 2009;34(6):1364‐75. - PubMed
Tang 2013 {published data only}
Verduri 2015 {published data only}
Wang 2016 {published data only}
-
- Wang JX, Zhang SM, Li XH, Zhang Y, Xu ZY, Cao B. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: a prospective randomized controlled trial. International Journal of Infectious Diseases 2016;48:40‐5. [DOI: 10.1016/j.ijid.2016.04.024] - DOI - PubMed
References to studies excluded from this review
Dharaniyadewi 2013 {published data only}
-
- Dharaniyadewi D, Lie KC, Sukmana N, Rumende CM. Effect of semi‐quantitative procalcitonin assay on the adequacy of empirical antibiotics and mortality in septic patients. Citical Care 2013;17(Suppl 4):P15.
Esposito 2012 {published data only}
-
- Esposito S, Tagliabue C, Picciolli I, Semino M, Sabatini C, Consolo S, et al. Procalcitonin measurements for guiding antibiotic treatment in pediatric pneumonia. Respiratory Medicine 2011;105(12):1939‐45. - PubMed
Heyland 2011 {published data only}
-
- Heyland DK, Johnson AP, Reynolds SC, Muscedere J. Procalcitonin for reduced antibiotic exposure in the critical care setting: a systematic review and an economic evaluation. Critical Care Medicine 2011;39(7):1792‐9. - PubMed
Jensen 2011 {published data only}
-
- Jensen JU, Hein L, Lundgren B, Bestle MH, Mohr TT, Andersen MH, et al. Procalcitonin‐guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Critical Care Medicine 2011;39(9):2048‐58. - PubMed
Jones 2007 {published data only}
-
- Jones AE, Fiechtl JF, Brown MD, Ballew JJ, Kline JA. Procalcitonin test in the diagnosis of bacteremia: a meta‐analysis. Annals of Emergency Medicine 2007;50(1):34‐41. - PubMed
Kook 2012 {published data only}
-
- Kook JL, Chao SR, Le J, Robinson PA. Impact of the use of procalcitonin assay in hospitalised patients with pneumonia at a community care hospital. Infection Control and Hospital Epidemiology 2012;33(4):424‐6. - PubMed
Liew 2011 {published data only}
-
- Liew YX, Chlebicki MP, Lee W, Hsu LY, Kwa AL. Use of procalcitonin (PCT) to guide discontinuation of antibiotic use in an unspecified sepsis is an antimicrobial stewardship program (ASP). European Journal of Clinical Microbiology and Infectious Diseases 2011;30:853‐5. - PubMed
Liu 2013 {published data only}
-
- Liu BH, Li HF, Lei Y, Zhao SX, Sun ML. Clinical significance of dynamic monitoring of procalcitonin in guiding the use of antibiotics in patients with sepsis in ICU. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2013;25(11):690‐3. - PubMed
Qu 2012 {published data only}
-
- Qu R, Ji Y, Ling Y, Ye CY, Yang SM, Liu YY, et al. Procalcitonin is a good tool to guide duration of antibiotic therapy in patients with severe acute pancreatitis. A randomized prospective single‐center controlled trial. Saudi Medical Journal 2012;33(4):382‐7. - PubMed
Saeed 2011 {published data only}
-
- Saeed K, Dryden M, Bourne S, Paget C, Proud A. Reduction in antibiotic use through procalcitonin testing in patients in the medical admission unit or intensive care unit with suspicion of infection. Journal of Hospital Infection 2011;78(4):289‐92. - PubMed
Schuetz 2010 {published data only}
-
- Schuetz P, Batschwaroff M, Dusemund F, Albrich W, Burgi U, Maurer M, et al. Effectiveness of a procalcitonin algorithm to guide antibiotic therapy in respiratory tract infections outside of study conditions: a post‐study survey. European Journal of Clinical Microbiology and Infectious Diseases 2010;29(3):269‐77. - PubMed
Simmonds 2005 {published data only}
-
- Simmonds MC, Higgins JP, Stewart LA, Tierney JF, Clarke MJ, Thompson SG. Meta‐analysis of individual patient data from randomized trials: a review of methods used in practice. Clinical Trials 2005;2(3):209‐17. - PubMed
Simon 2004 {published data only}
-
- Simon L, Gauvin F, Amre DK, Saint‐Louis P, Lacroix J. Serum procalcitonin and C‐reactive protein levels as markers of bacterial infection: a systematic review and meta‐analysis. Clinical Infectious Diseases 2004;39(2):206‐17. - PubMed
Stocker 2010 {published data only}
-
- Stocker M, Fontana M, Helou S, Wegscheider K, Berger TM. Use of procalcitonin‐guided decision‐making to shorten antibiotic therapy in suspected neonatal early‐onset sepsis: prospective randomized intervention trial. Neonatology 2010;97(2):165‐74. - PubMed
Tang 2007 {published data only}
-
- Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta‐analysis. Lancet Infectious Diseases 2007;7(3):210‐7. - PubMed
Tang 2009 {published data only}
-
- Tang H, Huang T, Jing J, Shen H, Cui W. Effect of procalcitonin‐guided treatment in patients with infections: a systematic review and meta‐analysis. Infection 2009;37(6):497‐507. - PubMed
Uzzan 2006 {published data only}
-
- Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta‐analysis. Critical Care Medicine 2006;34(7):1996‐2003. - PubMed
References to ongoing studies
NCT02130986 {unpublished data only}
-
- NCT02130986. Procalcitonin Antibiotic Consensus Trial (ProACT). clinicaltrials.gov/ct2/show/study/NCT02130986 First received: May 1, 2014.
NCT02261610 {unpublished data only}
-
- NCT02261610. Pulmonary Embolism and PCT. PE‐PCT Study. clinicaltrials.gov/ct2/show/NCT02261610 First received: September 5, 2014.
NCT02332577 {unpublished data only}
-
- NCT02332577. Study to compare the efficacy of pristinamycin (Pyostacine) versus amoxicillin in the treatment of acute community acquired pneumonia. clinicaltrials.gov/ct2/show/NCT02332577 First received: January 5, 2015.
NCT02440828 {unpublished data only}
-
- NCT02440828. Addition of tobramycin inhalation in the treatment of ventilator associated pneumonia (VAPORISE). clinicaltrials.gov/ct2/show/NCT02440828 First received: March 13, 2015.
NCT02787603 {unpublished data only}
-
- NCT02787603. Procalcitonin in Early Antibiotic Interruption in Patient With Bacterial Pulmonary infeCtion and Acute Heart Failure (EPICAD). clinicaltrials.gov/ct2/show/NCT02787603 First received: May 25, 2016.
NCT02862314 {unpublished data only}
-
- NCT02862314. PROcalcitonin Pneumonia/Pneumonitis Associated With ASPIration (PROPASPI). clinicaltrials.gov/ct2/show/NCT02862314 First received: July 29, 2016.
NCT02931409 {unpublished data only}
-
- NCT02931409. Intraoperative PEEP optimization: effects on postoperative pulmonary complications and inflammatory response. clinicaltrials.gov/ct2/show/NCT02931409 First received: October 5, 2016.
Additional references
Albrich 2012
-
- Albrich WC, Dusemund F, Bucher B, Meyer S, Thomann R, Kuhn F, et al. Effectiveness and safety of procalcitonin‐guided antibiotic therapy in lower respiratory tract infections in "real life": an international, multicenter post‐study survey (ProREAL). Archives of Internal Medicine 2012;172(9):715‐22. - PubMed
Arnold 2005
Atkins 2004
Balk 2017
Cals 2009
Doan 2014
Drozdov 2015
Evans 2002
-
- Evans AT, Husain S, Durairaj L, Sadowski LS, Charles‐Damte M, Wang Y. Azithromycin for acute bronchitis: a randomised, double‐blind, controlled trial. Lancet 2002;359(9318):1648‐54. - PubMed
Gonzales 1997
-
- Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA 1997;278(11):901‐4. - PubMed
Goossens 2005
-
- Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross‐national database study. Lancet 2005;365(9435):579‐87. - PubMed
GRADEpro GDT 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version (accessed April 2017). Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoare 2006
Hoeboer 2015
-
- Hoeboer SH, Geest PJ, Nieboer D, Groeneveld AB. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta‐analysis. Clinical Microbiology and Infection 2015;21(5):474‐81. - PubMed
Konstantinides 2008
-
- Konstantinides S. Clinical practice. Acute pulmonary embolism. New England Journal of Medicine 2008;359(26):2804‐13. - PubMed
Kumar 2006
-
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Critical Care Medicine 2006;34(6):1589‐96. - PubMed
Kumar 2009
-
- Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009;136(5):1237‐48. - PubMed
Kutz 2015
Kutz 2016
-
- Kutz K, Hausfater P, Oppert M, Alan M, Grolimund E, Gast C, et al. Comparison between B·R·A·H·M·S PCT direct, a new sensitive point‐of‐care testing device for rapid quantification of procalcitonin in emergency department patients and established reference methods ‐ a prospective multinational trial. Clinical Chemistry and Laboratory Medicine 2016;54(4):577‐84. - PubMed
Lawrence 2009
-
- Lawrence KL, Kollef MH. Antimicrobial stewardship in the intensive care unit: advances and obstacles. American Journal of Respiratory and Critical Care Medicine 2009;179(6):434‐8. - PubMed
Liberati 2009a
-
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology 2009;62(10):e1‐34. - PubMed
Liberati 2009b
Manzano 2010
-
- Manzano S, Bailey B, Girodias JB, Galetto‐Lacour A, Cousineau J, Delvin E. Impact of procalcitonin on the management of children aged 1 to 36 months presenting with fever without source: a randomized controlled trial. American Journal of Emergency Medicine 2010;28(6):647‐53. - PubMed
Meili 2015
-
- Meili M, Muller B, Kulkarni P, Schutz P. Management of patients with respiratory infections in primary care: procalcitonin, C‐reactive protein or both?. Expert Review of Respiratory Medicine 2015;9(5):587‐601. - PubMed
Meili 2016
Muller 2000
-
- Muller B, Becker KL, Schachinger H, Rickenbacher PR, Huber PR, Zimmerli W, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Critical Care Medicine 2000;28(4):977‐83. - PubMed
Muller 2001
-
- Muller B, Becker KL. Procalcitonin: how a hormone became a marker and mediator of sepsis. Swiss Medical Weekly 2001;131(41‐2):595‐602. - PubMed
Muller 2010
-
- Muller F, Christ‐Crain M, Bregenzer T, Krause M, Zimmerli W, Mueller B, et al. Procalcitonin levels predict bacteremia in patients with community‐acquired pneumonia: a prospective cohort trial. Chest 2010;138(1):121‐9. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sager 2017
Schuetz 2011a
-
- Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Archives of Internal Medicine 2011;171(15):1322‐31. - PubMed
Schuetz 2015
-
- Schuetz P, Aujesky D, Muller C, Muller B. Biomarker‐guided personalised emergency medicine for all ‐ hope for another hype?. Swiss Medical Weekly 2015;145:w14079. - PubMed
Schuetz 2016
Schuetz 2017
Spurling 2010
Stata 12.1 [Computer program]
-
- StataCorp LP. Stata Statistical Software: Release 12.1.. College Station (TX): StataCorp LP, 2005.
Stewart 2015
-
- Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred reporting items for systematic review and meta‐analyses of individual participant data: the PRISMA‐IPD Statement. JAMA 2015;313(16):1657‐65. - PubMed
Stojanovic 2017
-
- Stojanovic I, Schneider JE, Wei L, Hong Z, Keane C, Schuetz P. Economic evaluation of procalcitonin‐guided antibiotic therapy in acute respiratory infections: a Chinese hospital system perspective. Clinical Chemistry and Laboratory Medicine 2017;55(4):561‐70. - PubMed
Thompson 2001
-
- Thompson SG, Turner RM, Warn DE. Multilevel models for meta‐analysis, and their application to absolute risk differences. Statistical Methods in Medical Research 2001;10(6):375‐92. - PubMed
Turner 2000
-
- Turner RM, Omar RZ, Yang M, Goldstein H, Thompson SG. A multilevel model framework for meta‐analysis of clinical trials with binary outcomes. Statistics in Medicine 2000;19(24):3417‐32. - PubMed
van Vugt 2013
-
- Vugt SF, Broekhuizen BD, Lammens C, Zuithoff NP, Jong PA, Coenen S, et al. Use of serum C reactive protein and procalcitonin concentrations in addition to symptoms and signs to predict pneumonia in patients presenting to primary care with acute cough: diagnostic study. BMJ 2013;346:f2450. - PMC - PubMed
Wacker 2013
-
- Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta‐analysis. Lancet Infectious Diseases 2013;13(5):426‐35. - PubMed
Zaas 2014
-
- Zaas AK, Garner BH, Tsalik EL, Burke T, Woods CW, Ginsburg GS. The current epidemiology and clinical decisions surrounding acute respiratory infections. Trends in Molecular Medicine 2014;20(10):579‐88. - PubMed
References to other published versions of this review
Schuetz 2008
Schuetz 2010a
-
- Schuetz P, Albrich W, Christ‐Crain M, Chastre J, Mueller B. Procalcitonin for guidance of antibiotic therapy. Expert Review of Anti‐Infective Therapy 2010;8(5):575‐87. - PubMed
Schuetz 2011
-
- Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Archives of Internal Medicine 2011;171(15):1322‐31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

