Genes for asparagine metabolism in Lotus japonicus: differential expression and interconnection with photorespiration
- PMID: 29025409
- PMCID: PMC5639745
- DOI: 10.1186/s12864-017-4200-x
Genes for asparagine metabolism in Lotus japonicus: differential expression and interconnection with photorespiration
Abstract
Background: Asparagine is a very important nitrogen transport and storage compound in plants due to its high nitrogen/carbon ratio and stability. Asparagine intracellular concentration depends on a balance between asparagine biosynthesis and degradation. The main enzymes involved in asparagine metabolism are asparagine synthetase (ASN), asparaginase (NSE) and serine-glyoxylate aminotransferase (SGAT). The study of the genes encoding for these enzymes in the model legume Lotus japonicus is of particular interest since it has been proposed that asparagine is the principal molecule used to transport reduced nitrogen within the plant in most temperate legumes.
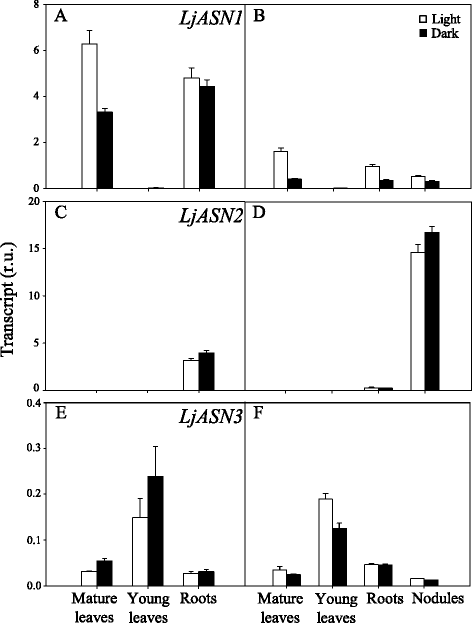
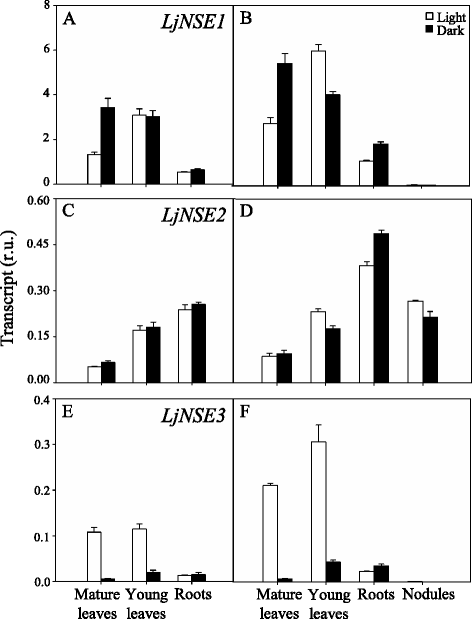
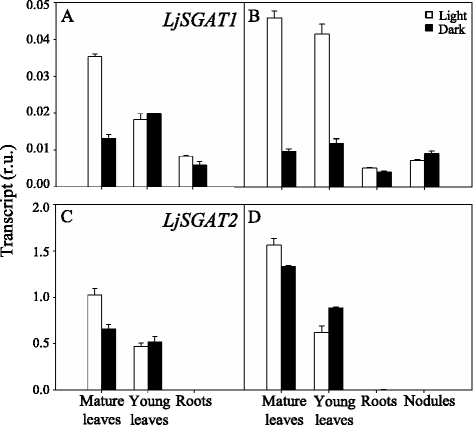
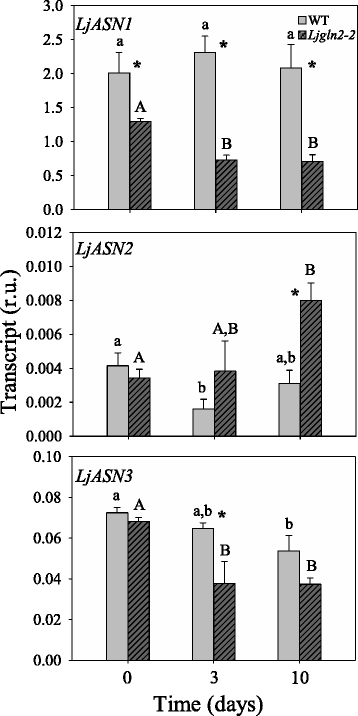
Results: A differential expression of genes encoding for several enzymes involved in asparagine metabolism was detected in L. japonicus. ASN is encoded by three genes, LjASN1 was the most highly expressed in mature leaves while LjASN2 expression was negligible and LjASN3 showed a low expression in this organ, suggesting that LjASN1 is the main gene responsible for asparagine synthesis in mature leaves. In young leaves, LjASN3 was the only ASN gene expressed although at low levels, while all the three genes encoding for NSE were highly expressed, especially LjNSE1. In nodules, LjASN2 and LjNSE2 were the most highly expressed genes, suggesting an important role for these genes in this organ. Several lines of evidence support the connection between asparagine metabolic genes and photorespiration in L. japonicus: a) a mutant plant deficient in LjNSE1 showed a dramatic decrease in the expression of the two genes encoding for SGAT; b) expression of the genes involved in asparagine metabolism is altered in a photorespiratory mutant lacking plastidic glutamine synthetase; c) a clustering analysis indicated a similar pattern of expression among several genes involved in photorespiratory and asparagine metabolism, indicating a clear link between LjASN1 and LjSGAT genes and photorespiration.
Conclusions: The results obtained in this paper indicate the existence of a differential expression of asparagine metabolic genes in L. japonicus and point out the crucial relevance of particular genes in different organs. Moreover, the data presented establish clear links between asparagine and photorespiratory metabolic genes in this plant.
Keywords: Asparaginase genes; Asparagine synthetase genes; Lotus japonicus; Serine-glyoxylate aminotransferase genes.
Conflict of interest statement
Ethics approval and consent to participate
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- do Amarante L, Lima JD, Sodek L. Growth and stress conditions cause similar changes in xylem amino acids for different legume species. Environ Exp Bot. 2006;58(1–3):123–129. doi: 10.1016/j.envexpbot.2005.07.002. - DOI
-
- Gaufichon L, Reisdorf-Cren M, Rothstein SJ, Chardon F, Suzuki A. Biological functions of asparagine synthetase in plants. Plant Sci. 2010;179(3):141–153. doi: 10.1016/j.plantsci.2010.04.010. - DOI
-
- Duff SMG. Asparagine synthetase. In: Mello JPF, editor. Amino acids in higher plants. CAB international. 2015. pp. 100–128.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

