Investigating Bordetella pertussis colonisation and immunity: protocol for an inpatient controlled human infection model
- PMID: 29025851
- PMCID: PMC5652574
- DOI: 10.1136/bmjopen-2017-018594
Investigating Bordetella pertussis colonisation and immunity: protocol for an inpatient controlled human infection model
Abstract
Introduction: We summarise an ethically approved protocol for the development of an experimental human challenge colonisation model. Globally Bordetella pertussis is one of the leading causes of vaccine-preventable death. Many countries have replaced whole cell vaccines with acellular vaccines over the last 20 years during which pertussis appears to be resurgent in a number of countries in the developed world that boast high immunisation coverage. The acellular vaccine provides relatively short-lived immunity and, in contrast to whole cell vaccines, may be less effective against colonisation and subsequent transmission. To improve vaccine strategies, a greater understanding of human B. pertussis colonisation is required. This article summarises a protocol and does not contain any results.
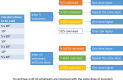
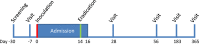
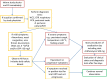
Methods and analysis: A controlled human colonisation model will be developed over two phases. In phase A, a low dose of the inoculum will be given intranasally to healthy participants. This dose will be escalated or de-escalated until colonisation is achieved in approximately 70% (95% CI 47% to 93%) of the exposed volunteers without causing disease. The colonisation period, shedding and exploratory immunology will be assessed during a 17-day inpatient stay and follow-up over 1 year. The dose of inoculum that achieves 70% colonisation will then be confirmed in phase B, comparing healthy participants exposed to B. pertussis with a control group receiving a sham inoculum.
Ethics and dissemination: This study has been approved by the ethical committee reference: 17/SC/0006, 24 February 2017. Findings will be published in peer-reviewed open access journals as soon as possible.
Keywords: bordetella pertussis; colonisation; human challenge study; vaccine development; whooping cough.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: HDG hasreceived sponsorship from Abbvie to attend a clinical paediatric rheumatology course. SF acts on behalf of the University ofSouthampton/University Hospital Southampton NHS Foundation trust as chief and principal investigator for clinical trials Sponsored by vaccine andantimicrobial manufacturers but receives no personal payments for the work.
Figures
Similar articles
-
Do we need a new vaccine to control the re-emergence of pertussis?Trends Microbiol. 2014 Feb;22(2):49-52. doi: 10.1016/j.tim.2013.11.007. Trends Microbiol. 2014. PMID: 24485284
-
Human Immune Responses to Pertussis Vaccines.Adv Exp Med Biol. 2019;1183:99-113. doi: 10.1007/5584_2019_406. Adv Exp Med Biol. 2019. PMID: 31342460
-
Roads to the development of improved pertussis vaccines paved by immunology.Pathog Dis. 2015 Nov;73(8):ftv067. doi: 10.1093/femspd/ftv067. Epub 2015 Sep 6. Pathog Dis. 2015. PMID: 26347400 Free PMC article. Review.
-
Comparison of Three Whole-Cell Pertussis Vaccines in the Baboon Model of Pertussis.Clin Vaccine Immunol. 2015 Nov 11;23(1):47-54. doi: 10.1128/CVI.00449-15. Print 2016 Jan. Clin Vaccine Immunol. 2015. PMID: 26561389 Free PMC article.
-
T-cell immune responses to Bordetella pertussis infection and vaccination.Pathog Dis. 2015 Oct;73(7):ftv051. doi: 10.1093/femspd/ftv051. Epub 2015 Aug 4. Pathog Dis. 2015. PMID: 26242279 Review.
Cited by
-
Controlled human malaria infection: overview and potential application in the evaluation of transmission-blocking interventions in malaria-endemic areas.Malar J. 2025 Feb 1;24(1):33. doi: 10.1186/s12936-025-05277-x. Malar J. 2025. PMID: 39893367 Free PMC article. Review.
-
Th1 polarization in Bordetella pertussis vaccine responses is maintained through a positive feedback loop.Nat Commun. 2025 Apr 1;16(1):3132. doi: 10.1038/s41467-025-58460-8. Nat Commun. 2025. PMID: 40169665 Free PMC article.
-
Age and Primary Vaccination Background Influence the Plasma Cell Response to Pertussis Booster Vaccination.Vaccines (Basel). 2022 Jan 18;10(2):136. doi: 10.3390/vaccines10020136. Vaccines (Basel). 2022. PMID: 35214595 Free PMC article.
-
Intranasal inoculation with Bordetella pertussis confers protection without inducing classical whooping cough in baboons.Curr Res Microb Sci. 2021 Oct 9;2:100072. doi: 10.1016/j.crmicr.2021.100072. eCollection 2021 Dec. Curr Res Microb Sci. 2021. PMID: 34841362 Free PMC article.
-
Th1 polarization in Bordetella pertussis vaccine responses is maintained through a positive feedback loop.bioRxiv [Preprint]. 2024 Oct 17:2024.08.05.606623. doi: 10.1101/2024.08.05.606623. bioRxiv. 2024. Update in: Nat Commun. 2025 Apr 01;16(1):3132. doi: 10.1038/s41467-025-58460-8. PMID: 39149302 Free PMC article. Updated. Preprint.
References
-
- Public Health England. Factsheet Pertussis: Public Health England, 2016. https://www.gov.uk/government/uploads/system/uploads/attachment_data/fil....
-
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–71. 10.1016/S0140-6736(14)61682-2 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
