Effect of erdosteine on the rate and duration of COPD exacerbations: the RESTORE study
- PMID: 29025888
- PMCID: PMC5678897
- DOI: 10.1183/13993003.00711-2017
Effect of erdosteine on the rate and duration of COPD exacerbations: the RESTORE study
Abstract
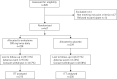
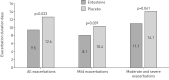
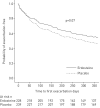
Oxidative stress contributes to chronic obstructive pulmonary disease (COPD) exacerbations and antioxidants can decrease exacerbation rates, although we lack data about the effect of such drugs on exacerbation duration.The RESTORE (Reducing Exacerbations and Symptoms by Treatment with ORal Erdosteine in COPD) study was a prospective randomised, double-blind, placebo-controlled study, enrolling patients aged 40-80 years with Global Initiative for Chronic Obstructive Lung Disease stage II/III. Patients received erdosteine 300 mg twice daily or placebo added to usual COPD therapy for 12 months. The primary outcome was the number of acute exacerbations during the study.In the pre-specified intention-to-treat population of 445 patients (74% male; mean age 64.8 years, forced expiratory volume in 1 s 51.8% predicted) erdosteine reduced the exacerbation rate by 19.4% (0.91 versus 1.13 exacerbations·patient-1·year-1 for erdosteine and placebo, respectively; p=0.01), due to an effect on mild events; the reduction in the rate of mild exacerbations was 57.1% (0.23 versus 0.54 exacerbations·patient-1·year-1 for erdosteine and placebo, respectively; p=0.002). No significant difference was observed in the rate of moderate and severe exacerbations (0.68 versus 0.59 exacerbations·patient-1·year-1 for erdosteine and placebo, respectively; p=0.054) despite a trend in favour of the comparison group. Erdosteine decreased the exacerbation duration irrespective of event severity by 24.6% (9.55 versus 12.63 days for erdosteine and placebo, respectively; p=0.023). Erdosteine significantly improved subject and physician subjective severity scores (p=0.022 and p=0.048, respectively), and reduced the use of reliever medication (p<0.001), but did not affect the St George's Respiratory Questionnaire score or the time to first exacerbation.In patients with COPD, erdosteine can reduce both the rate and duration of exacerbations. The percentage of patients with adverse events was similar in both the placebo and erdosteine treatment groups.
Trial registration: ClinicalTrials.gov NCT01032304.
Copyright ©ERS 2017.
Conflict of interest statement
Conflict of interest: Disclosures can be found alongside this article at erj.ersjournals.com
Figures

Comment in
-
Mucolytics for COPD: negotiating a slippery slope towards proof of efficacy.Eur Respir J. 2017 Oct 12;50(4):1701465. doi: 10.1183/13993003.01465-2017. Print 2017 Oct. Eur Respir J. 2017. PMID: 29025882 No abstract available.
References
-
- Vogelmeier CF, Criner GJ, Martinez FT, et al.Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Eur Respir J 2017; 49: 170021. - PubMed
-
- Wilkinson TM, Donaldson GC, Hurst JR, et al.Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 169: 1298–1303. - PubMed
-
- Seemungal TA, Donaldson GC, Paul EA, et al.Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157: 1418–1422. - PubMed
-
- Vestbo J, Edwards LD, Scanlon PD, et al.Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 2011; 365: 1184–1192. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
