Comparative profiling of HLA-DR and HLA-DQ associated factor VIII peptides presented by monocyte-derived dendritic cells
- PMID: 29025906
- PMCID: PMC5777204
- DOI: 10.3324/haematol.2017.175083
Comparative profiling of HLA-DR and HLA-DQ associated factor VIII peptides presented by monocyte-derived dendritic cells
Abstract
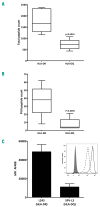
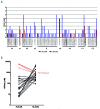
The development of anti-factor VIII antibodies is a major complication of the treatment of patients with hemophilia A. Generation of high affinity anti-factor VIII antibodies is dependent on help provided by CD4+ T cells that recognize factor VIII-derived peptides presented on class II major histocompatibility complex on the surface of antigen-presenting cells. In order to identify the immune-dominant epitopes that can be presented to CD4+ T cells, we previously developed a mass spectrometry-based method to identify factor VIII-derived peptides that are presented on human leukocyte antigen (HLA)-DR. In the present work, we compared the repertoire of FVIII-derived peptide presented on HLA-DR and HLA-DQ. Monocyte-derived dendritic cells from nine HLA-typed healthy donors were pulsed with recombinant factor VIII. HLA-DR and HLA-DQ molecules were purified using monoclonal antibodies. Our data show that HLA-DQ and HLA-DR present a similar repertoire of factor VIII-derived peptides. However, the number of peptides associated with HLA-DQ was lower than that with HLA-DR. We also identified a peptide, within the acidic a3 domains of factor VIII, which is presented with higher frequency on HLA-DQ. Interestingly, this peptide was found to have a higher predicted affinity for HLA-DQ than for HLA-DR. Taken together, our data suggest that HLA-DQ participates in the presentation of factor VIII peptides, thereby contributing to the development of inhibitory antibodies in a proportion of patients with severe hemophilia A.
Copyright© 2018 Ferrata Storti Foundation.
Figures


References
-
- Fijnvandraat K, Cnossen MH, Leebeek FWG, Peters M. Diagnosis and management of haemophilia. BMJ. 2012;344:e2707. - PubMed
-
- Franchini M, Tagliaferri A, Mengoli C, Cruciani M. Cumulative inhibitor incidence in previously untreated patients with severe hemophilia A treated with plasma-derived versus recombinant factor VIII concentrates: a critical systematic review. Crit Rev Oncol Hematol. 2012;81(1):82–93. - PubMed
-
- Gouw SC, van den Berg HM, Fischer K, et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: the RODIN study. Blood. 2013;121(20):4046–4055. - PubMed
-
- Hay CRM, Palmer B, Chalmers E, et al. Incidence of factor VIII inhibitors throughout life in severe hemophilia A in the United Kingdom. Blood. 2011;117(23):6367–6370. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

