Effective purifying selection in ancient asexual oribatid mites
- PMID: 29026136
- PMCID: PMC5638860
- DOI: 10.1038/s41467-017-01002-8
Effective purifying selection in ancient asexual oribatid mites
Abstract
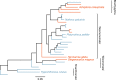
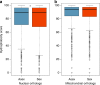
Sex is beneficial in the long term because it can prevent mutational meltdown through increased effectiveness of selection. This idea is supported by empirical evidence of deleterious mutation accumulation in species with a recent transition to asexuality. Here, we study the effectiveness of purifying selection in oribatid mites which have lost sex millions of years ago and diversified into different families and species while reproducing asexually. We compare the accumulation of deleterious nonsynonymous and synonymous mutations between three asexual and three sexual lineages using transcriptome data. Contrasting studies of young asexual lineages, we find evidence for strong purifying selection that is more effective in asexual as compared to sexual oribatid mite lineages. Our results suggest that large populations likely sustain effective purifying selection and facilitate the escape of mutational meltdown in the absence of sex. Thus, sex per se is not a prerequisite for the long-term persistence of animal lineages.Asexual reproduction is thought to be an evolutionary dead end in eukaryotes because deleterious mutations will not be purged effectively. Here, Brandt and colleagues show that anciently asexual oribatid mites in fact have reduced accumulation of deleterious mutations compared to their sexual relatives.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Bell, G. The Masterpiece of Nature: The Evolution and Genetics of Sexuality 1–635 (Croom Helm Ltd., 1982).
-
- Williams, G. C. Sex and Evolution 1–200 (Princeton University Press, 1975).
-
- Maynard Smith, J. The Evolution of Sex 1–222 (Cambridge University Press, 1978).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

