Engineering and physical sciences in oncology: challenges and opportunities
- PMID: 29026204
- PMCID: PMC5683724
- DOI: 10.1038/nrc.2017.83
Engineering and physical sciences in oncology: challenges and opportunities
Erratum in
-
Publisher Correction: Engineering and physical sciences in oncology: challenges and opportunities.Nat Rev Cancer. 2018 Nov;18(11):720. doi: 10.1038/s41568-018-0072-x. Nat Rev Cancer. 2018. PMID: 30337715 Free PMC article.
Abstract
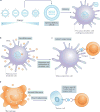
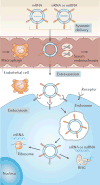
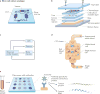
The principles of engineering and physics have been applied to oncology for nearly 50 years. Engineers and physical scientists have made contributions to all aspects of cancer biology, from quantitative understanding of tumour growth and progression to improved detection and treatment of cancer. Many early efforts focused on experimental and computational modelling of drug distribution, cell cycle kinetics and tumour growth dynamics. In the past decade, we have witnessed exponential growth at the interface of engineering, physics and oncology that has been fuelled by advances in fields including materials science, microfabrication, nanomedicine, microfluidics, imaging, and catalysed by new programmes at the National Institutes of Health (NIH), including the National Institute of Biomedical Imaging and Bioengineering (NIBIB), Physical Sciences in Oncology, and the National Cancer Institute (NCI) Alliance for Nanotechnology. Here, we review the advances made at the interface of engineering and physical sciences and oncology in four important areas: the physical microenvironment of the tumour and technological advances in drug delivery; cellular and molecular imaging; and microfluidics and microfabrication. We discussthe research advances, opportunities and challenges for integrating engineering and physical sciences with oncology to develop new methods to study, detect and treat cancer, and we also describe the future outlook for these emerging areas.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 2014;16:321–346. Comprehensive review of the role of physical forces in tumour progression and therapy for those new to the fields of engineering and physical sciences in oncology. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

