Efferocytosis of dying cells differentially modulate immunological outcomes in tumor microenvironment
- PMID: 29027226
- PMCID: PMC8359679
- DOI: 10.1111/imr.12587
Efferocytosis of dying cells differentially modulate immunological outcomes in tumor microenvironment
Abstract
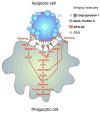
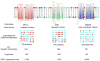
Programmed cell death (apoptosis) is an integral part of tissue homeostasis in complex organisms, allowing for tissue turnover, repair, and renewal while simultaneously inhibiting the release of self antigens and danger signals from apoptotic cell-derived constituents that can result in immune activation, inflammation, and autoimmunity. Unlike cells in culture, the physiological fate of cells that die by apoptosis in vivo is their rapid recognition and engulfment by phagocytic cells (a process called efferocytosis). To this end, apoptotic cells express specific eat-me signals, such as externalized phosphatidylserine (PS), that are recognized in a specific context by receptors to initiate signaling pathways for engulfment. The importance of carefully regulated recognition and clearance pathways is evident in the spectrum of inflammatory and autoimmune disorders caused by defects in PS receptors and signaling molecules. However, in recent years, several additional cell death pathways have emerged, including immunogenic cell death, necroptosis, pyroptosis, and netosis that interweave different cell death pathways with distinct innate and adaptive responses from classical apoptosis that can shape long-term host immunity. In this review, we discuss the role of different cell death pathways in terms of their immune potential outcomes specifically resulting in specific cell corpse/phagocyte interactions (phagocytic synapses) that impinge on host immunity, with a main emphasis on tolerance and cancer immunotherapy.
Keywords: cell death; immunogenic cell death; phosphatidylserine; tolerance; tumor immunity.
© 2017 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
No potential conflicts of interest were disclosed by the authors.
Figures
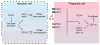


References
-
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. - PubMed
-
- Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. - PubMed
-
- Voll RE, Herrmann M, Roth EA, et al. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

