Geobiological feedbacks and the evolution of thermoacidophiles
- PMID: 29028004
- PMCID: PMC5739016
- DOI: 10.1038/ismej.2017.162
Geobiological feedbacks and the evolution of thermoacidophiles
Abstract
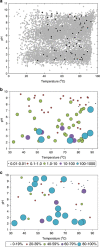
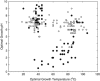
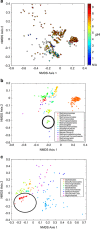
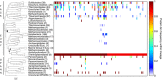
Oxygen-dependent microbial oxidation of sulfur compounds leads to the acidification of natural waters. How acidophiles and their acidic habitats evolved, however, is largely unknown. Using 16S rRNA gene abundance and composition data from 72 hot springs in Yellowstone National Park, Wyoming, we show that hyperacidic (pH<3.0) hydrothermal ecosystems are dominated by a limited number of archaeal lineages with an inferred ability to respire O2. Phylogenomic analyses of 584 existing archaeal genomes revealed that hyperacidophiles evolved independently multiple times within the Archaea, each coincident with the emergence of the ability to respire O2, and that these events likely occurred in the recent evolutionary past. Comparative genomic analyses indicated that archaeal thermoacidophiles from independent lineages are enriched in similar protein-coding genes, consistent with convergent evolution aided by horizontal gene transfer. Because the generation of acidic environments and their successful habitation characteristically require O2, these results suggest that thermoacidophilic Archaea and the acidity of their habitats co-evolved after the evolution of oxygenic photosynthesis. Moreover, it is likely that dissolved O2 concentrations in thermal waters likely did not reach levels capable of sustaining aerobic thermoacidophiles and their acidifying activity until ~0.8 Ga, when present day atmospheric levels were reached, a time period that is supported by our estimation of divergence times for archaeal thermoacidophilic clades.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

