Impact of Pneumococcal Vaccination on Pneumonia Hospitalizations and Related Costs in Ontario: A Population-Based Ecological Study
- PMID: 29029063
- PMCID: PMC5848302
- DOI: 10.1093/cid/cix850
Impact of Pneumococcal Vaccination on Pneumonia Hospitalizations and Related Costs in Ontario: A Population-Based Ecological Study
Abstract
Background: In Ontario, Canada, pneumococcal conjugate vaccine (PCV) was approved for infants in 2001 and became part of the publicly funded routine immunization schedule in 2005. We assessed the population-level impact of PCV on pneumonia hospitalizations and related costs.
Methods: We used the difference-in-differences approach to evaluate the impact of pneumococcal vaccination on pneumonia hospitalizations and related costs, using nonpneumonia hospitalization as the control condition. We extracted monthly hospitalization costs, stratified by age group, from population-based health administrative data between April 1992 and March 2014. The study period was divided into 5 intervals: prevaccine period, availability of 7-valent PCV (PCV7) for private purchase, public funding for PCV7, replacement of PCV7 with 10-valent PCV (PCV10), and replacement of PCV10 with 13-valent PCV (PCV13).
Results: A total of 1063700 pneumonia hospitalizations were recorded during the study period. In the vaccine-eligible age group, pneumonia hospitalizations declined by 34% (95% confidence interval, 32%-37%), 38% (32%-43%), and 45% (40%-51%) and hospitalization-related costs declined by 38% (25%-51%), 39% (33%-45%), and 46% (41%-52%) after public funding for PCV7, PCV10, and PCV13, respectively. Pneumonia hospitalizations and related costs also declined substantially for PCV-ineligible older children and elderly persons (aged >65 years).
Conclusions: Our results suggest that the publicly funded PCV immunization program is responsible for substantial reductions in pneumonia hospitalizations and related healthcare costs, among both young children eligible for publicly funded vaccination and other age groups not included in the publicly funded program.
Keywords: Streptococcus pneumoniae; cost; hospitalization; pneumococcal conjugate vaccine; pneumonia.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
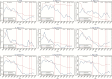
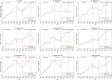
Comment in
-
Ecological Fallacy, Nonspecific Outcomes, and the Attribution of Disproportionate Vaccine Benefits.Clin Infect Dis. 2018 May 17;66(11):1817-1818. doi: 10.1093/cid/ciy014. Clin Infect Dis. 2018. PMID: 29741599 Free PMC article. No abstract available.
References
-
- Rudnick W, Liu Z, Shigayeva A et al. Pneumococcal vaccination programs and the burden of invasive pneumococcal disease in Ontario, Canada, 1995–2011. Vaccine 2013; 31:5863–71. - PubMed
-
- Black S, Shinefield H, Fireman B et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J 2000; 19:187–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

