Thyroid Hormone Metabolism Defects in a Mouse Model of SBP2 Deficiency
- PMID: 29029094
- PMCID: PMC5711384
- DOI: 10.1210/en.2017-00618
Thyroid Hormone Metabolism Defects in a Mouse Model of SBP2 Deficiency
Abstract
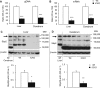
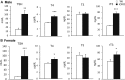
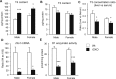
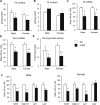
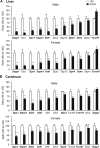
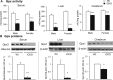
Selenocysteine insertion sequence binding protein 2 (SBP2) is an essential factor in selenoprotein synthesis. Patients with SBP2 defects have a characteristic thyroid phenotype and additional manifestations such as growth delay, male infertility, impaired motor coordination, and developmental delay. The thyroid phenotype has become pathognomonic for this defect, and putative deficiencies in the iodothyronine deiodinases selenoenzymes have been implicated. To investigate the role of SBP2 and selenoproteins in thyroid physiology and answer questions raised by the human syndrome, we generated a tamoxifen-inducible Sbp2 conditional knockout (iCKO) mouse model. These Sbp2-deficient mice have high serum thyroxine (T4), thyrotropin, and reverse triiodothyronine (T3), similar to the human phenotype of SBP2 deficiency, whereas serum T3 is normal. Their liver T4 and T3 content reflect the serum levels, and deiodinase 1 expression and enzymatic activity were decreased. In contrast, brain T3 content is decreased, indicative of local hypothyroidism, confirmed by the decreased expression of the thyroid hormone (TH) positively regulated gene hairless. Interestingly, the cerebrum T4 content did not parallel the high serum T4 levels, and the expression of TH transporters was decreased. Deiodinase 2 enzymatic activity and deiodinase 3 expression were decreased in cerebrum. The expression and/or activity of other selenoproteins were decreased in brain, liver, and serum, thus demonstrating a global deficiency in selenoprotein synthesis. Sbp2 iCKO mice replicate the thyroid phenotype of SBP2 deficiency and represent an important tool to advance our understanding of the role of SBP2 in thyroid homeostasis and for investigating selenoprotein biology relevant to human disease.
Copyright © 2017 Endocrine Society.
Figures


References
-
- Dumitrescu AM, Liao XH, Abdullah MS, Lado-Abeal J, Majed FA, Moeller LC, Boran G, Schomburg L, Weiss RE, Refetoff S. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet. 2005;37(11):1247–1252. - PubMed
-
- Schoenmakers E, Agostini M, Mitchell C, Schoenmakers N, Papp L, Rajanayagam O, Padidela R, Ceron-Gutierrez L, Doffinger R, Prevosto C, Luan J, Montano S, Lu J, Castanet M, Clemons N, Groeneveld M, Castets P, Karbaschi M, Aitken S, Dixon A, Williams J, Campi I, Blount M, Burton H, Muntoni F, O’Donovan D, Dean A, Warren A, Brierley C, Baguley D, Guicheney P, Fitzgerald R, Coles A, Gaston H, Todd P, Holmgren A, Khanna KK, Cooke M, Semple R, Halsall D, Wareham N, Schwabe J, Grasso L, Beck-Peccoz P, Ogunko A, Dattani M, Gurnell M, Chatterjee K. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J Clin Invest. 2010;120(12):4220–4235. - PMC - PubMed
-
- Azevedo MF, Barra GB, Naves LA, Ribeiro Velasco LF, Godoy Garcia Castro P, de Castro LC, Amato AA, Miniard A, Driscoll D, Schomburg L, de Assis Rocha Neves F. Selenoprotein-related disease in a young girl caused by nonsense mutations in the SBP2 gene. J Clin Endocrinol Metab. 2010;95(8):4066–4071. - PubMed
-
- Hamajima T, Mushimoto Y, Kobayashi H, Saito Y, Onigata K. Novel compound heterozygous mutations in the SBP2 gene: characteristic clinical manifestations and the implications of GH and triiodothyronine in longitudinal bone growth and maturation. Eur J Endocrinol. 2012;166(4):757–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

