No Clinical Benefit of Empirical Antimicrobial Therapy for Pediatric Diarrhea in a High-Usage, High-Resistance Setting
- PMID: 29029149
- PMCID: PMC5850041
- DOI: 10.1093/cid/cix844
No Clinical Benefit of Empirical Antimicrobial Therapy for Pediatric Diarrhea in a High-Usage, High-Resistance Setting
Abstract
Background: Pediatric diarrheal disease presents a major public health burden in low- to middle-income countries. The clinical benefits of empirical antimicrobial treatment for diarrhea are unclear in settings that lack reliable diagnostics and have high antimicrobial resistance (AMR).
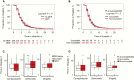
Methods: We conducted a prospective multicenter cross-sectional study of pediatric patients hospitalized with diarrhea containing blood and/or mucus in Ho Chi Minh City, Vietnam. Clinical parameters, including disease outcome and treatment, were measured. Shigella, nontyphoidal Salmonella (NTS), and Campylobacter were isolated from fecal samples, and their antimicrobial susceptibility profiles were determined. Statistical analyses, comprising log-rank tests and accelerated failure time models, were performed to assess the effect of antimicrobials on disease outcome.
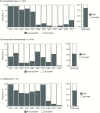
Results: Among 3166 recruited participants (median age 10 months; interquartile range, 6.5-16.7 months), one-third (1096 of 3166) had bloody diarrhea, and 25% (793 of 3166) were culture positive for Shigella, NTS, or Campylobacter. More than 85% of patients (2697 of 3166) were treated with antimicrobials; fluoroquinolones were the most commonly administered antimicrobials. AMR was highly prevalent among the isolated bacteria, including resistance against fluoroquinolones and third-generation cephalosporins. Antimicrobial treatment and multidrug resistance status of the infecting pathogens were found to have no significant effect on outcome. Antimicrobial treatment was significantly associated with an increase in the duration of hospitalization with particular groups of diarrheal diseases.
Conclusions: In a setting with high antimicrobial usage and high AMR, our results imply a lack of clinical benefit for treating diarrhea with antimicrobials; adequately powered randomized controlled trials are required to assess the role of antimicrobials for diarrhea.
Keywords: Campylobacter; Shigella; antimicrobial resistance; disease outcome; fluoroquinolones; multidrug resistance; nontyphoidal Salmonella; pediatric diarrhea.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Comment in
-
Overutilization of Antibiotics in Children With Diarrhea: First Do No Harm.Clin Infect Dis. 2018 Feb 1;66(4):512-513. doi: 10.1093/cid/cix845. Clin Infect Dis. 2018. PMID: 29028953 No abstract available.
References
-
- Scallan E, Mahon BE, Hoekstra RM, Griffin PM. Estimates of illnesses, hospitalizations, and deaths caused by major bacterial enteric pathogens in young children in the United States. Pediatr Infect Dis J 2012; 32:217–21. - PubMed
-
- Murray CJL, Vos T, Lozano R et al. . Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2197–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

