Sleepless Night and Day, the Plight of Progressive Supranuclear Palsy
- PMID: 29029214
- PMCID: PMC6251530
- DOI: 10.1093/sleep/zsx154
Sleepless Night and Day, the Plight of Progressive Supranuclear Palsy
Abstract
Objectives: To elucidate the unique sleep and waking characteristics in progressive supranuclear palsy (PSP), a neurodegenerative disease associated with motor deficits and dementia that largely affects the brainstem and thalamic regions.
Methods: A total of 20 PSP and 16 healthy older adult controls participated in this study. The participants underwent an overnight polysomnography and multiple sleep latency test (MSLT) the following day. Prior to the MSLT last trial, they were asked to complete the Stanford Sleepiness Scale. Data were assessed for measures of latency to sleep onset, sleep duration, waking, and sleep staging during the night. Mean sleep latency, a measure of daytime sleepiness, sleep onset rapid eye movement (REM) periods, and microsleeps were studied with the MSLT. Spectral analysis of wake electroencephalogram (EEG) was performed for 30-second periods at the start of each MSLT trial.
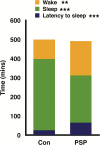
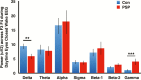
Results: PSP took significantly longer time to fall asleep (p < .001), slept less during the night (p ≤ .001), and had more wake after sleep onset than controls (p ≤ .001). PSP had less N2 sleep (p < .05) and N3 sleep (p < .05), and REM sleep (p < .001) than controls. During the MSLT, PSP took significantly longer to fall asleep (p < .001), did not have microsleeps when they remained awake throughout the assessment periods, but were subjectively sleepier than controls (p < .05). Gamma power was increased during wake EEG in PSP (p < .01).
Conclusions: Sleep/waking regulation and REM sleep regulation are disrupted in PSP, leading to profound sleep deprivation without recuperation. Our findings suggest a diminished homeostatic sleep drive in PSP. This hyperaroused state is unique and is a severely disabling feature of PSP.
Keywords: homeostatic sleep drive; hyperarousal; multiple sleep latency test; neurodegenerative disease; spectral analysis.
© Sleep Research Society 2017. Published by Oxford University Press on behalf of the Sleep Research Society. All rights reserved. For permissions, please e-mail journals.permissions@oup.com.
Figures


References
-
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005; 437(7063): 1257–1263. - PubMed
-
- McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007; 8(4): 302–330. - PubMed
-
- Litvan I, Agid Y, Calne D et al. . Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele–Richardson–Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996; 47(1): 1–9. - PubMed
-
- Williams DR, Holton JL, Strand C et al. . Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson’s syndrome. Brain. 2007; 130(Pt 6): 1566–1576. - PubMed
-
- Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA. Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol. 2010; 23(4): 394–400. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

