An Adjuvanted, Postfusion F Protein-Based Vaccine Did Not Prevent Respiratory Syncytial Virus Illness in Older Adults
- PMID: 29029260
- PMCID: PMC5853767
- DOI: 10.1093/infdis/jix503
An Adjuvanted, Postfusion F Protein-Based Vaccine Did Not Prevent Respiratory Syncytial Virus Illness in Older Adults
Abstract
Background: Respiratory syncytial virus (RSV) is an important cause of illness in older adults. This study assessed efficacy of a vaccine for prevention of RSV-associated acute respiratory illness (ARI), defined by specified symptoms with virologic confirmation.
Methods: This phase 2b study evaluated RSV postfusion F protein (120 µg) with glucopyranosyl lipid adjuvant (5 µg) in 2% stable emulsion. Subjects aged ≥60 years were randomly assigned at a ratio of 1:1 to receive vaccine or placebo (all received inactivated influenza vaccine). Ill subjects recorded symptoms and provided blood and nasal swab samples.
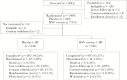
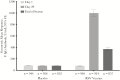
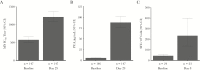
Results: In the per-protocol population (n = 1894), the incidence of RSV-associated ARI occurring ≥14 days after dosing was 1.7% and 1.6% in the vaccine and placebo groups, respectively, for a vaccine efficacy (VE) of -7.1% (90% confidence interval [CI], -106.9%-44.3%). Efficacy was not observed in secondary analyses that included seroresponse to nonvaccine RSV antigens (VE, 8.9%; 90% CI, -28.5%-35.4%) or symptoms combined with seroresponse (VE, 10.0%; 90% CI, -45.4%-44.4%). On day 29, 92.9% of vaccinees had an anti-F immunoglobulin G antibody seroresponse. Overall, 48.5% and 30.9% of RSV vaccine recipients reported local and systemic solicited symptoms, respectively.
Conclusion: The RSV vaccine was immunogenic but did not protect older adults from RSV illness.
Clinical trials registration: NCT02508194.
Keywords: Adjuvant; clinical trial; efficacy; respiratory syncytial virus; subunit; vaccine.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Comment in
-
Vaccine Prevention of Respiratory Syncytial Virus Infection in Older Adults: The Work Continues.J Infect Dis. 2017 Dec 12;216(11):1334-1336. doi: 10.1093/infdis/jix504. J Infect Dis. 2017. PMID: 29029125 Free PMC article. No abstract available.
References
-
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. - PubMed
-
- Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 1991; 163:693–8. - PubMed
-
- Falsey AR, Walsh EE. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J Infect Dis 1998; 177:463–6. - PubMed
-
- Malloy AMW, Falsey AR, Ruckwardt TJ. Consequences of immature and senescent immune responses for infection with respiratory syncytial virus. In: Anderson LJ, Graham BS, eds. Challenges and opportunities for respiratory syncytial virus vaccines. Berlin, Heidelberg: Springer Berlin Heidelberg, 2013:211–231. - PubMed
-
- Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis 2004; 189:233–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

