Efficacy and safety of delafloxacin compared with vancomycin plus aztreonam for acute bacterial skin and skin structure infections: a Phase 3, double-blind, randomized study
- PMID: 29029278
- PMCID: PMC5890686
- DOI: 10.1093/jac/dkx329
Efficacy and safety of delafloxacin compared with vancomycin plus aztreonam for acute bacterial skin and skin structure infections: a Phase 3, double-blind, randomized study
Abstract
Background: Delafloxacin is an investigational anionic fluoroquinolone in development for oral or intravenous administration for the treatment of infections caused by Gram-positive (including MRSA), Gram-negative, atypical and anaerobic organisms.
Objectives: To establish the non-inferiority of delafloxacin compared with vancomycin plus aztreonam for the treatment of acute bacterial skin and skin structure infections and to compare the safety of the two antimicrobials.
Patients and methods: A Phase 3, multicentre, randomized, double-blind, active-controlled study with 660 patients compared delafloxacin 300 mg or vancomycin 15 mg/kg plus aztreonam 2 g each administered twice daily intravenously for 5-14 days. Non-inferiority was evaluated by objective response (≥20% erythema reduction) at 48-72 h after initiation of study drug, investigator subjective assessment of outcome and microbiological responses. Clinical Trials Registration: NCT01811732. EudraCT number: 2012-001767-71.
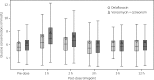
Results: In the ITT analysis set, the objective response was 78.2% in the delafloxacin arm and 80.9% in the vancomycin/aztreonam arm (mean treatment difference, -2.6%; 95% CI, -8.78% to 3.57%). Investigator-assessed cure was similar between the two groups at follow-up (52.0% versus 50.5%) and late follow-up (70.4% versus 66.6%). Bacterial eradication of MRSA was 100% and 98.5% in the delafloxacin group and the vancomycin/aztreonam group, respectively. Frequency of treatment-emergent adverse events in the delafloxacin and vancomycin/aztreonam groups was similar. Treatment-emergent adverse events leading to study drug discontinuation were higher in the vancomycin/aztreonam group compared with the delafloxacin group (4.3% versus 0.9%).
Conclusions: Delafloxacin, an anionic fluoroquinolone, was statistically non-inferior to vancomycin/aztreonam at 48-72 h following the start of therapy and was well tolerated as monotherapy in the treatment of acute bacterial skin and skin structure infections.
© The Author 2017. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy.
Figures



References
-
- Stevens DL, Bisno AL, Chambers HF. et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59: e10–52. - PubMed
-
- Stevens DL, Bisno AL, Chambers HF. et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41: 1373–406. - PubMed
-
- Barie PS, Wilson SE.. Impact of evolving epidemiology on treatments for complicated skin and skin structure infections: the surgical perspective. J Am Coll Surg 2015; 220: 105–16. - PubMed
-
- Guillamet CV, Kollef MH.. How to stratify patients at risk for resistant bugs in skin and soft tissue infections? Curr Opin Infect Dis 2016; 29: 116–23. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

