Inhibition of intestinal ascorbic acid uptake by lipopolysaccharide is mediated via transcriptional mechanisms
- PMID: 29030247
- PMCID: PMC5732882
- DOI: 10.1016/j.bbamem.2017.10.010
Inhibition of intestinal ascorbic acid uptake by lipopolysaccharide is mediated via transcriptional mechanisms
Abstract
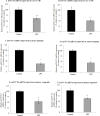
Ascorbic acid (AA) accumulation in intestinal epithelial cells is an active transport process mainly mediated by two sodium-dependent vitamin C transporters (SVCT-1 and SVCT-2). To date, little is known about the effect of gut microbiota generated lipopolysaccharide (LPS) on intestinal absorption of water-soluble vitamins. Therefore, the objective of this study was to investigate the effects of bacterially-derived LPS on AA homeostasis in enterocytes using Caco-2 cells, mouse intestine and intestinal enteroids models. Pre-treating Caco-2 cells and mice with LPS led to a significant decrease in carrier-mediated AA uptake. This inhibition was associated with a significant reduction in SVCT-1 and SVCT-2 protein, mRNA, and hnRNA expression. Furthermore, pre-treating enteroids with LPS also led to a marked decrease in SVCT-1 and SVCT-2 protein and mRNA expression. Inhibition of SVCT-1 and SVCT-2 occurred at least in part at the transcriptional level as promoter activity of SLC23A1 and SLC23A2 was attenuated following LPS treatment. Subsequently, we examined the protein and mRNA expression levels of HNF1α and Sp1 transcription factors, which are needed for basal SLC23A1 and SLC23A2 promoter activity, and found that they were significantly decreased in the LPS treated Caco-2 cells and mouse jejunum; this was reflected on level of the observed reduction in the interaction of these transcription factors with their respective promoters in Caco-2 cells treated with LPS. Our findings indicate that LPS inhibits intestinal carrier- mediated AA uptake by down regulating the expression of both vitamin C transporters and transcriptional regulation of SLC23A1 and SLC23A2 genes.
Keywords: Enteroids; SVCT-1; SVCT-2; Transport; Uptake; Vitamin C.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
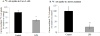


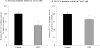

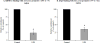
Similar articles
-
Tumor necrosis factor alpha reduces intestinal vitamin C uptake: a role for NF-κB-mediated signaling.Am J Physiol Gastrointest Liver Physiol. 2018 Aug 1;315(2):G241-G248. doi: 10.1152/ajpgi.00071.2018. Epub 2018 Apr 6. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29631379 Free PMC article.
-
Molecular mechanism(s) involved in differential expression of vitamin C transporters along the intestinal tract.Am J Physiol Gastrointest Liver Physiol. 2017 Apr 1;312(4):G340-G347. doi: 10.1152/ajpgi.00369.2016. Epub 2016 Dec 8. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 27932501 Free PMC article.
-
Enterotoxigenic Escherichia coli heat labile enterotoxin inhibits intestinal ascorbic acid uptake via a cAMP-dependent NF-κB-mediated pathway.Am J Physiol Gastrointest Liver Physiol. 2019 Jan 1;316(1):G55-G63. doi: 10.1152/ajpgi.00259.2018. Epub 2018 Oct 4. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 30285481 Free PMC article.
-
Regulation of vitamin C transport.Annu Rev Nutr. 2005;25:105-25. doi: 10.1146/annurev.nutr.25.050304.092647. Annu Rev Nutr. 2005. PMID: 16011461 Review.
-
The sodium-dependent ascorbic acid transporter family SLC23.Mol Aspects Med. 2013 Apr-Jun;34(2-3):436-54. doi: 10.1016/j.mam.2012.12.002. Mol Aspects Med. 2013. PMID: 23506882 Review.
Cited by
-
Salmonella Typhimurium Infection Reduces the Ascorbic Acid Uptake in the Intestine.Mediators Inflamm. 2023 Jan 17;2023:2629262. doi: 10.1155/2023/2629262. eCollection 2023. Mediators Inflamm. 2023. PMID: 36704315 Free PMC article.
-
The Variable Nature of Vitamin C-Does It Help When Dealing with Coronavirus?Antioxidants (Basel). 2022 Jun 24;11(7):1247. doi: 10.3390/antiox11071247. Antioxidants (Basel). 2022. PMID: 35883738 Free PMC article. Review.
-
Tumor necrosis factor alpha reduces intestinal vitamin C uptake: a role for NF-κB-mediated signaling.Am J Physiol Gastrointest Liver Physiol. 2018 Aug 1;315(2):G241-G248. doi: 10.1152/ajpgi.00071.2018. Epub 2018 Apr 6. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29631379 Free PMC article.
-
Intestinal microbiota as a route for micronutrient bioavailability.Curr Opin Endocr Metab Res. 2021 Sep 4;20:100285. doi: 10.1016/j.coemr.2021.100285. eCollection 2021 Oct. Curr Opin Endocr Metab Res. 2021. PMID: 34676307 Free PMC article.
-
The Contribution of Plasma and Brain Vitamin C on Age and Gender-Related Cognitive Differences: A Mini-Review of the Literature.Front Integr Neurosci. 2020 Aug 21;14:47. doi: 10.3389/fnint.2020.00047. eCollection 2020. Front Integr Neurosci. 2020. PMID: 32973470 Free PMC article. Review.
References
-
- Packer L, Fuchs J. Vitamin C in health and disease. Marcel Dekker Inc.; New York, NY: 1997.
-
- Alcantara-Martos T, Delgado-Martinez AD, Vega MV, Carrascal MT, Munuera-Martinez L. Effect of vitamin C on fracture healing in elderly osteogenic disorder shionogi rats. J Bone joint Surg Br. 2007;89:402–407. - PubMed
-
- Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES) Am J Clin Nutr. 2009;90:1252–1263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

