Single-cell RNA-seq reveals a distinct transcriptome signature of aneuploid hematopoietic cells
- PMID: 29030335
- PMCID: PMC5746166
- DOI: 10.1182/blood-2017-08-803353
Single-cell RNA-seq reveals a distinct transcriptome signature of aneuploid hematopoietic cells
Abstract
Cancer cells frequently exhibit chromosomal abnormalities. Specific cytogenetic aberrations often are predictors of outcome, especially in hematologic neoplasms, such as monosomy 7 in myeloid malignancies. The functional consequences of aneuploidy at the cellular level are difficult to assess because of a lack of convenient markers to distinguish abnormal from diploid cells. We performed single-cell RNA sequencing (scRNA-seq) to study hematopoietic stem and progenitor cells from the bone marrow of 4 healthy donors and 5 patients with bone marrow failure and chromosome gain or loss. In total, transcriptome sequences were obtained from 391 control cells and 588 cells from patients. We characterized normal hematopoiesis as binary differentiation from stem cells to erythroid and myeloid-lymphoid pathways. Aneuploid cells were distinguished from diploid cells in patient samples by computational analyses of read fractions and gene expression of individual chromosomes. We confirmed assignment of aneuploidy to individual cells quantitatively, by copy-number variation, and qualitatively, by loss of heterozygosity. When we projected patients' single cells onto the map of normal hematopoiesis, diverse patterns were observed, broadly reflecting clinical phenotypes. Patients' monosomy 7 cells showed downregulation of genes involved in immune response and DNA damage checkpoint and apoptosis pathways, which may contribute to the clonal expansion of monosomy 7 cells with accumulated gene mutations. scRNA-seq is a powerful technique through which to infer the functional consequences of chromosome gain and loss and explore gene targets for directed therapy.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


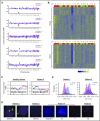

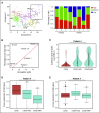


Comment in
-
Single-cell dissection of monosomy 7 syndromes.Blood. 2017 Dec 21;130(25):2693-2695. doi: 10.1182/blood-2017-11-811935. Blood. 2017. PMID: 29269530 No abstract available.
References
-
- Arber DA, Orazi A, Hasserjian R, et al. . The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. - PubMed
-
- Klatte T, Rao PN, de Martino M, et al. . Cytogenetic profile predicts prognosis of patients with clear cell renal cell carcinoma. J Clin Oncol. 2009;27(5):746-753. - PubMed
-
- Panani AD, Roussos C. Cytogenetic and molecular aspects of lung cancer. Cancer Lett. 2006;239(1):1-9. - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

