Transcriptome and Functional Profile of Cardiac Myocytes Is Influenced by Biological Sex
- PMID: 29030402
- PMCID: PMC5679409
- DOI: 10.1161/CIRCGENETICS.117.001770
Transcriptome and Functional Profile of Cardiac Myocytes Is Influenced by Biological Sex
Abstract
Background: Although cardiovascular disease is the primary killer of women in the United States, women and female animals have traditionally been omitted from research studies. In reports that do include both sexes, significant sexual dimorphisms have been demonstrated in development, presentation, and outcome of cardiovascular disease. However, there is little understanding of the mechanisms underlying these observations. A more thorough understanding of sex-specific cardiovascular differences both at baseline and in disease is required to effectively consider and treat all patients with cardiovascular disease.
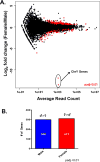
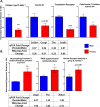
Methods and results: We analyzed contractility in the whole rat heart, adult rat ventricular myocytes (ARVMs), and myofibrils from both sexes of rats and observed functional sex differences at all levels. Hearts and ARVMs from female rats displayed greater fractional shortening than males, and female ARVMs and myofibrils took longer to relax. To define factors underlying these functional differences, we performed an RNA sequencing experiment on ARVMs from male and female rats and identified ≈600 genes were expressed in a sexually dimorphic manner. Further analysis revealed sex-specific enrichment of signaling pathways and key regulators. At the protein level, female ARVMs exhibited higher protein kinase A activity, consistent with pathway enrichment identified through RNA sequencing. In addition, activating the protein kinase A pathway diminished the contractile sexual dimorphisms previously observed.
Conclusions: These data support the notion that sex-specific gene expression differences at baseline influence cardiac function, particularly through the protein kinase A pathway, and could potentially be responsible for differences in cardiovascular disease presentation and outcomes.
Keywords: cardiovascular diseases; gene expression; myocytes, cardiac; rats; sequence analysis, RNA; sex differences.
© 2017 American Heart Association, Inc.
Figures



Comment in
-
Sex Determines Cardiac Myocyte Stretch and Relaxation.Circ Cardiovasc Genet. 2017 Oct;10(5):e001950. doi: 10.1161/CIRCGENETICS.117.001950. Circ Cardiovasc Genet. 2017. PMID: 29030406 Free PMC article. No abstract available.
References
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2015 update: A report from the american heart association. Circulation. 2015;131:e29–322. - PubMed
-
- Consideration of sex as a biological variable in nih-funded research. 2015
-
- Regitz-Zagrosek V. Therapeutic implications of the gender-specific aspects of cardiovascular disease. Nat Rev Drug Discov. 2006;5:425–438. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

