Lipids at membrane contact sites: cell signaling and ion transport
- PMID: 29030479
- PMCID: PMC5666614
- DOI: 10.15252/embr.201744331
Lipids at membrane contact sites: cell signaling and ion transport
Abstract
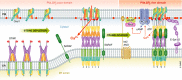
Communication between organelles is essential to coordinate cellular functions and the cell's response to physiological and pathological stimuli. Organellar communication occurs at membrane contact sites (MCSs), where the endoplasmic reticulum (ER) membrane is tethered to cellular organelle membranes by specific tether proteins and where lipid transfer proteins and cell signaling proteins are located. MCSs have many cellular functions and are the sites of lipid and ion transfer between organelles and generation of second messengers. This review discusses several aspects of MCSs in the context of lipid transfer, formation of lipid domains, generation of Ca2+ and cAMP second messengers, and regulation of ion transporters by lipids.
Keywords: contact sites; ion transport; lipid transfer; signaling.
Published 2017. This article is a U.S. Government work and is in the public domain in the USA.
Figures


References
-
- Berridge MJ (2016) The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev 96: 1261–1296 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

