Intrinsic aerobic capacity governs the associations between gut microbiota composition and fat metabolism age-dependently in rat siblings
- PMID: 29030493
- PMCID: PMC5814668
- DOI: 10.1152/physiolgenomics.00081.2017
Intrinsic aerobic capacity governs the associations between gut microbiota composition and fat metabolism age-dependently in rat siblings
Abstract
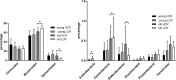
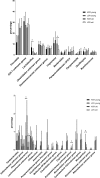
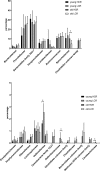
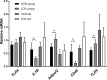
Host genetic factors affecting the gut microbiome play an important role in obesity, yet limited attention has been paid on the host genetic factors linked to physical fitness in modifying the microbiome. This study determined whether sibling-matched pairs of rats selectively bred for high (HCR) and low (LCR) aerobic capacity differ in their microbiome age-dependently and which taxa associate with differential in metabolism. Several taxa in young adult rats (hereafter young) linked to inherited aerobic capacity, while in older adult (hereafter old) rats most of the differences between the lines associated with body weight. Despite the absence of weight differential between LCR and HCR when young, the LCR microbiome contained more Actinobacteria, Veillonellaceae, Coriobacteriaceae, Phascolarctobacterium, and Ruminococcus; taxa previously linked to obesity. This raises the question whether the microbiome contributes to the later development of obesity in LCR. Age-related differences were detected in almost all taxa in both rat lines. The young HCR measured higher for serum glycerol and free fatty-acids and lower for cholesterol, HDL, LDL, and triglycerides than LCR. The old HCR differed from the old LCR by lower LDL. Several metabolites, including LDL, are associated age and genetic background-dependently with the microbiome, which might explain the metabolic differences between the lines. While old lines did not differ in visceral adipose tissue gene expression, the young HCR expressed more inflammatory genes than LCR, and several taxa including Proteobacteria associated with these genes. In conclusion, intrinsic aerobic capacity governs the microbiome, which may influence body weight, metabolism, and gene expression.
Keywords: gene expression; gut microbiota; intrinsic aerobic capacity; lipid metabolism.
Figures


Similar articles
-
Gut microbiota are linked to increased susceptibility to hepatic steatosis in low-aerobic-capacity rats fed an acute high-fat diet.Am J Physiol Gastrointest Liver Physiol. 2016 Jul 1;311(1):G166-79. doi: 10.1152/ajpgi.00065.2016. Epub 2016 Jun 10. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27288420 Free PMC article.
-
Intrinsic aerobic capacity impacts susceptibility to acute high-fat diet-induced hepatic steatosis.Am J Physiol Endocrinol Metab. 2014 Aug 15;307(4):E355-64. doi: 10.1152/ajpendo.00093.2014. Epub 2014 Jun 24. Am J Physiol Endocrinol Metab. 2014. PMID: 24961240 Free PMC article.
-
Physical Activity Differentially Affects the Cecal Microbiota of Ovariectomized Female Rats Selectively Bred for High and Low Aerobic Capacity.PLoS One. 2015 Aug 24;10(8):e0136150. doi: 10.1371/journal.pone.0136150. eCollection 2015. PLoS One. 2015. PMID: 26301712 Free PMC article.
-
Dietary lipids, gut microbiota and lipid metabolism.Rev Endocr Metab Disord. 2019 Dec;20(4):461-472. doi: 10.1007/s11154-019-09512-0. Rev Endocr Metab Disord. 2019. PMID: 31707624 Free PMC article. Review.
-
Genetic and microbiome influence on lipid metabolism and dyslipidemia.Physiol Genomics. 2018 Feb 1;50(2):117-126. doi: 10.1152/physiolgenomics.00053.2017. Epub 2017 Dec 20. Physiol Genomics. 2018. PMID: 29341867 Free PMC article. Review.
Cited by
-
The Role of Gut Microbiota in Different Types of Physical Activity and Their Intensity: Systematic Review and Meta-Analysis.Sports (Basel). 2024 Aug 14;12(8):221. doi: 10.3390/sports12080221. Sports (Basel). 2024. PMID: 39195597 Free PMC article. Review.
-
Vertical selection for nuclear and mitochondrial genomes shapes gut microbiota and modifies risks for complex diseases.Physiol Genomics. 2020 Jan 1;52(1):1-14. doi: 10.1152/physiolgenomics.00089.2019. Epub 2019 Nov 25. Physiol Genomics. 2020. PMID: 31762410 Free PMC article.
-
Harnessing the strategy of metagenomics for exploring the intestinal microecology of sable (Martes zibellina), the national first-level protected animal.AMB Express. 2020 Sep 18;10(1):169. doi: 10.1186/s13568-020-01103-6. AMB Express. 2020. PMID: 32945998 Free PMC article.
-
Physiological and Biochemical Effects of Intrinsically High and Low Exercise Capacities Through Multiomics Approaches.Front Physiol. 2019 Sep 18;10:1201. doi: 10.3389/fphys.2019.01201. eCollection 2019. Front Physiol. 2019. PMID: 31620020 Free PMC article.
-
Six-Week Endurance Exercise Alters Gut Metagenome That Is not Reflected in Systemic Metabolism in Over-weight Women.Front Microbiol. 2018 Oct 3;9:2323. doi: 10.3389/fmicb.2018.02323. eCollection 2018. Front Microbiol. 2018. PMID: 30337914 Free PMC article.
References
-
- Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, Zhang M, Oh PL, Nehrenberg D, Hua K, Kachman SD, Moriyama EN, Walter J, Peterson DA, Pomp D. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci USA 107: 18933–18938, 2010. doi:10.1073/pnas.1007028107. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

