Pharmacological HIF-inhibition attenuates postoperative adhesion formation
- PMID: 29030625
- PMCID: PMC5640636
- DOI: 10.1038/s41598-017-13638-z
Pharmacological HIF-inhibition attenuates postoperative adhesion formation
Abstract
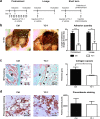
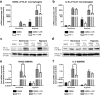
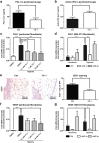
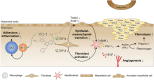
Peritoneal adhesions represent a common complication of abdominal surgery, and tissue hypoxia is a main determinant in adhesion formation. Reliable therapeutic options to reduce peritoneal adhesions are scarce. We investigated whether the formation of postsurgical adhesions can be affected by pharmacological interference with hypoxia-inducible factors (HIFs). Mice were treated with a small molecule HIF-inhibitor, YC-1 (3-[5'-Hydroxymethyl-2'-furyl]-1-benzyl-indazole), or vehicle three days before and seven days after induction of peritoneal adhesions or, alternatively, once during induction of peritoneal adhesions. Pretreatment or single intraperitoneal lavage with YC-1 significantly reduced postoperative adhesion formation without prompting systemic adverse effects. Expression analyses of cytokines in peritoneal tissue and fluid and in vitro assays applying macrophages and peritoneal fibroblasts indicated that this effect was cooperatively mediated by various putatively HIF-1α-dependent mechanisms, comprising attenuated pro-inflammatory activation of macrophages, impaired recruitment and activation of peritoneal fibroblasts, mitigated epithelial-mesenchymal-transition (EMT), as well as enhanced fibrinolysis and impaired angiogenesis. Thus, this study identifies prevention of postsurgical peritoneal adhesions as a novel and promising field for the application of HIF inhibitors in clinical practice.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

