Lubricin binds cartilage proteins, cartilage oligomeric matrix protein, fibronectin and collagen II at the cartilage surface
- PMID: 29030641
- PMCID: PMC5640667
- DOI: 10.1038/s41598-017-13558-y
Lubricin binds cartilage proteins, cartilage oligomeric matrix protein, fibronectin and collagen II at the cartilage surface
Abstract
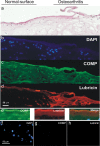
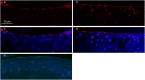
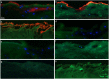
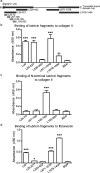
Lubricin, a heavily O-glycosylated protein, is essential for boundary lubrication of articular cartilage. Strong surface adherence of lubricin is required given the extreme force it must withstand. Disulfide bound complexes of lubricin and cartilage oligomeric matrix protein (COMP) have recently been identified in arthritic synovial fluid suggesting they may be lost from the cartilage surface in osteoarthritis and inflammatory arthritis. This investigation was undertaken to localise COMP-lubricin complexes within cartilage and investigate if other cartilage proteins are involved in anchoring lubricin to the joint. Immunohistochemical analysis of human cartilage biopsies showed lubricin and COMP co-localise to the cartilage surface. COMP knockout mice, however, presented with a lubricin layer on the articular cartilage leading to the further investigation of additional lubricin binding mechanisms. Proximity ligation assays (PLA) on human cartilage biopsies was used to localise additional lubricin binding partners and demonstrated that lubricin bound COMP, but also fibronectin and collagen II on the cartilage surface. Fibronectin and collagen II binding to lubricin was confirmed and characterised by solid phase binding assays with recombinant lubricin fragments. Overall, COMP, fibronectin and collagen II bind lubricin, exposed on the articular cartilage surface suggesting they may be involved in maintaining essential boundary lubrication.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

