Pooled efficacy and safety of eslicarbazepine acetate as add-on treatment in patients with focal-onset seizures: Data from four double-blind placebo-controlled pivotal phase III clinical studies
- PMID: 29030894
- PMCID: PMC5813188
- DOI: 10.1111/cns.12765
Pooled efficacy and safety of eslicarbazepine acetate as add-on treatment in patients with focal-onset seizures: Data from four double-blind placebo-controlled pivotal phase III clinical studies
Abstract
Purpose: Pooled evaluation of the key efficacy and safety profile of eslicarbazepine acetate (ESL) added-on to stable antiepileptic therapy in adults with focal-onset seizures.
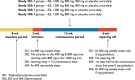
Methods: Data from 1703 patients enrolled in four phase III double-blind, randomized, placebo-controlled studies were pooled and analyzed. Following a 2 week titration period, ESL was administered at 400 mg, 800 mg, and 1200 mg once-daily doses for 12 weeks (maintenance period). Pooled efficacy variable was standardized (/4 weeks) seizure frequency (SSF) analyzed over the maintenance period as reduction in absolute and relative SSF and proportion of responders (≥50% reduction in SSF). Pooled safety was analyzed by means of adverse events and clinical laboratory assessments.
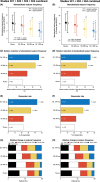
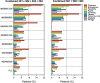
Results: SSF was significantly reduced with ESL 800 mg (P < 0.0001) and 1200 mg (P < 0.0001) compared to placebo. Median relative reduction in SSF was 33.4% for ESL 800 mg and 37.8% for 1200 mg (placebo: 17.6%), and responder rate was 33.8% and 43.1% (placebo: 22.2%). ESL was more efficacious than placebo regardless of gender, geographical region, epilepsy duration, age at time of diagnosis, seizure type, and type of concomitant antiepileptic drugs (AED). Incidence of adverse events (AEs) and AEs leading to discontinuation was dose dependent. Most common AEs (>10% patients) were dizziness, somnolence, and nausea. The incidence of treatment-emergent AEs (dizziness, somnolence, ataxia, vomiting, and nausea) was lower in patients who began taking ESL 400 mg (followed by 400 mg increments to 800 or 1200 mg) than in those who began taking ESL 600 mg or 800 mg.
Conclusions: Once-daily ESL 800 mg and 1200 mg showed consistent results across all efficacy and safety endpoints, independent of study population characteristics and type of concomitant AEDs. Treatment initiated with ESL 400 mg followed by 400 mg increments to 800 or 1200 mg provides optimal balance of efficacy and tolerability.
Keywords: adjunctive therapy; adults; antiepileptic drugs; eslicarbazepine acetate; focal-onset seizures; refractory epilepsy.
© 2017 The Authors. CNS Neuroscience & Therapeutics Published by John Wiley & Sons Ltd.
Conflict of interest statement
C. Elger, M. Koepp, E. Trinka (reports speakers honoraria and consultancy fees from Eisai, Everpharma, Medtronics, Bial, Newbridge, UCB Pharma, Boehringer, and his institution received grants from Biogen Idec, Eisai, Red Bull, and Merck. He or his institution received grants from European Union, FWF Österreichischer Fond zur Wissenschaftsförderung, Bundesministerium für Wissenschaft und Forschung, and the Jubiläumsfond der Österreichischen Nationalbank, outside the submitted work), V. Villanueva, J. Chaves, E. Ben‐Menachen, P. A. Kowacs, and A. Gil‐Nagel, have received research grants from BIAL, the sponsor of the studies. J. Moreira, H. Gama, J.F. Rocha, and P. Soares‐da‐Silva were employees of BIAL at the time of the studies.
Figures
References
-
- Ben‐Menachem E, Gabbai AA, Hufnagel A, Maia J, Almeida L, Soares‐da‐Silva P. Eslicarbazepine acetate as adjunctive therapy in adult patients with partial epilepsy. Epilepsy Res. 2010;89:278‐285. - PubMed
-
- Elger C, Halasz P, Maia J, Almeida L, Soares‐da‐Silva P. Efficacy and safety of eslicarbazepine acetate as adjunctive treatment in adults with refractory partial‐onset seizures: a randomized, double‐blind, placebo‐controlled, parallel‐group phase III study. Epilepsia. 2009;50:454‐463. - PubMed
-
- Gil‐Nagel A, Lopes‐Lima J, Almeida L, Maia J, Soares‐da‐Silva P. Efficacy and safety of 800 and 1200 mg eslicarbazepine acetate as adjunctive treatment in adults with refractory partial‐onset seizures. Acta Neurol Scand. 2009;120:281‐287. - PubMed
-
- EMA . CHMP Assessment Report for Zebinix. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_asses... Accessed July 12, 2016.: 2009 Contract No.: 304525.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

