Angiopoietin-like protein 4 is an exercise-induced hepatokine in humans, regulated by glucagon and cAMP
- PMID: 29031727
- PMCID: PMC5641605
- DOI: 10.1016/j.molmet.2017.06.018
Angiopoietin-like protein 4 is an exercise-induced hepatokine in humans, regulated by glucagon and cAMP
Abstract
Objective: Angiopoietin-like protein-4 (ANGPTL4) is a circulating protein that is highly expressed in liver and implicated in regulation of plasma triglyceride levels. Systemic ANGPTL4 increases during prolonged fasting and is suggested to be secreted from skeletal muscle following exercise.
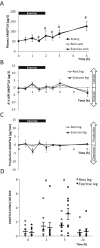
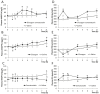
Methods: We investigated the origin of exercise-induced ANGPTL4 in humans by measuring the arterial-to-venous difference over the leg and the hepato-splanchnic bed during an acute bout of exercise. Furthermore, the impact of the glucagon-to-insulin ratio on plasma ANGPTL4 was studied in healthy individuals. The regulation of ANGPTL4 was investigated in both hepatic and muscle cells.
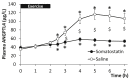
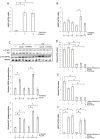
Results: The hepato-splanchnic bed, but not the leg, contributed to exercise-induced plasma ANGPTL4. Further studies using hormone infusions revealed that the glucagon-to-insulin ratio is an important regulator of plasma ANGPTL4 as elevated glucagon in the absence of elevated insulin increased plasma ANGPTL4 in resting subjects, whereas infusion of somatostatin during exercise blunted the increase of both glucagon and ANGPTL4. Moreover, activation of the cAMP/PKA signaling cascade let to an increase in ANGPTL4 mRNA levels in hepatic cells, which was prevented by inhibition of PKA. In humans, muscle ANGPTL4 mRNA increased during fasting, with only a marginal further induction by exercise. In human muscle cells, no inhibitory effect of AMPK activation could be demonstrated on ANGPTL4 expression.
Conclusions: The data suggest that exercise-induced ANGPTL4 is secreted from the liver and driven by a glucagon-cAMP-PKA pathway in humans. These findings link the liver, insulin/glucagon, and lipid metabolism together, which could implicate a role of ANGPTL4 in metabolic diseases.
Keywords: Diabetes; Insulin; Liver; Muscle; Myokine.
Copyright © 2017 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures


References
-
- Kersten S., Mandard S., Tan N.S., Escher P., Metzger D., Chambon P. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. Journal of Biological Chemistry. 2000;275:28488–28493. - PubMed
-
- Koster A., Chao Y.B., Mosior M., Ford A., Gonzalez-DeWhitt P.A., Hale J.E. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 2005;146:4943–4950. - PubMed
-
- Yau M.H., Wang Y., Lam K.S., Zhang J., Wu D., Xu A. A highly conserved motif within the NH2-terminal coiled-coil domain of angiopoietin-like protein 4 confers its inhibitory effects on lipoprotein lipase by disrupting the enzyme dimerization. Journal of Biological Chemistry. 2009;284:11942–11952. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

