The DNA Inflammasome in Human Myeloid Cells Is Initiated by a STING-Cell Death Program Upstream of NLRP3
- PMID: 29033128
- PMCID: PMC5901709
- DOI: 10.1016/j.cell.2017.09.039
The DNA Inflammasome in Human Myeloid Cells Is Initiated by a STING-Cell Death Program Upstream of NLRP3
Abstract
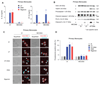
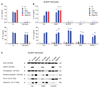
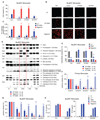
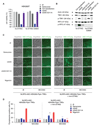
Detection of cytosolic DNA constitutes a central event in the context of numerous infectious and sterile inflammatory conditions. Recent studies have uncovered a bipartite mode of cytosolic DNA recognition, in which the cGAS-STING axis triggers antiviral immunity, whereas AIM2 triggers inflammasome activation. Here, we show that AIM2 is dispensable for DNA-mediated inflammasome activation in human myeloid cells. Instead, detection of cytosolic DNA by the cGAS-STING axis induces a cell death program initiating potassium efflux upstream of NLRP3. Forward genetics identified regulators of lysosomal trafficking to modulate this cell death program, and subsequent studies revealed that activated STING traffics to the lysosome, where it triggers membrane permeabilization and thus lysosomal cell death (LCD). Importantly, the cGAS-STING-NLRP3 pathway constitutes the default inflammasome response during viral and bacterial infections in human myeloid cells. We conclude that targeting the cGAS-STING-LCD-NLRP3 pathway will ameliorate pathology in inflammatory conditions that are associated with cytosolic DNA sensing.
Keywords: AIM2; Caspase-1; DNA; IL-1β; NLRP3; STING; cGAS; inflammasome; lysosomal cell death.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

