Oxysterol Restraint of Cholesterol Synthesis Prevents AIM2 Inflammasome Activation
- PMID: 29033131
- PMCID: PMC5693620
- DOI: 10.1016/j.cell.2017.09.029
Oxysterol Restraint of Cholesterol Synthesis Prevents AIM2 Inflammasome Activation
Abstract
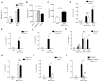
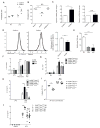
Type I interferon restrains interleukin-1β (IL-1β)-driven inflammation in macrophages by upregulating cholesterol-25-hydroxylase (Ch25h) and repressing SREBP transcription factors. However, the molecular links between lipid metabolism and IL-1β production remain obscure. Here, we demonstrate that production of 25-hydroxycholesterol (25-HC) by macrophages is required to prevent inflammasome activation by the DNA sensor protein absent in melanoma 2 (AIM2). We find that in response to bacterial infection or lipopolysaccharide (LPS) stimulation, macrophages upregulate Ch25h to maintain repression of SREBP2 activation and cholesterol synthesis. Increasing macrophage cholesterol content is sufficient to trigger IL-1β release in a crystal-independent but AIM2-dependent manner. Ch25h deficiency results in cholesterol-dependent reduced mitochondrial respiratory capacity and release of mitochondrial DNA into the cytosol. AIM2 deficiency rescues the increased inflammasome activity observed in Ch25h-/-. Therefore, activated macrophages utilize 25-HC in an anti-inflammatory circuit that maintains mitochondrial integrity and prevents spurious AIM2 inflammasome activation.
Keywords: 25-hydroxycholesterol; AIM2; Ch25h; IL-1β; SREBP; cholesterol; inflammasome; macrophage; mitochondria; oxysterol.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
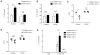




References
-
- Campos CBL, Paim BA, Cosso RG, Castilho RF, Rottenberg H, Vercesi AE. Method for monitoring of mitochondrial cytochrome C release during cell death: Immunodetection of Cytochrome C by flow cytometry after selective permeabilization of the plasma membrane. Cytometry. 2006;69:515–523. - PubMed
-
- Chen CH, Cheng YC. Delayed cytotoxicity and selective loss of mitochondrial DNA in cells treated with the anti-human immunodeficiency virus compound 2”,3-“dideoxycytidine. J Biol Chem. 1989;264:11934–11937. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

