Natively Unfolded FG Repeats Stabilize the Structure of the Nuclear Pore Complex
- PMID: 29033133
- PMCID: PMC5992322
- DOI: 10.1016/j.cell.2017.09.033
Natively Unfolded FG Repeats Stabilize the Structure of the Nuclear Pore Complex
Abstract
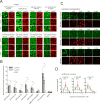
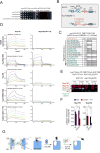
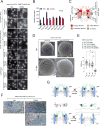
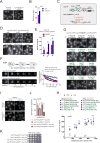
Nuclear pore complexes (NPCs) are ∼100 MDa transport channels assembled from multiple copies of ∼30 nucleoporins (Nups). One-third of these Nups contain phenylalanine-glycine (FG)-rich repeats, forming a diffusion barrier, which is selectively permeable for nuclear transport receptors that interact with these repeats. Here, we identify an additional function of FG repeats in the structure and biogenesis of the yeast NPC. We demonstrate that GLFG-containing FG repeats directly bind to multiple scaffold Nups in vitro and act as NPC-targeting determinants in vivo. Furthermore, we show that the GLFG repeats of Nup116 function in a redundant manner with Nup188, a nonessential scaffold Nup, to stabilize critical interactions within the NPC scaffold needed for late steps of NPC assembly. Our results reveal a previously unanticipated structural role for natively unfolded GLFG repeats as Velcro to link NPC subcomplexes and thus add a new layer of connections to current models of the NPC architecture.
Keywords: FG repeats; Intrinsically disordered domains; Nuclear envelope; Nuclear pore biogenesis; Nuclear pore complex; Nuclear pore structure; Protein interactions.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



References
-
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. The molecular architecture of the nuclear pore complex. Nature. 2007;450:695–701. - PubMed
-
- Allen NP, Huang L, Burlingame A, Rexach M. Proteomic analysis of nucleoporin interacting proteins. The Journal of biological chemistry. 2001;276:29268–29274. - PubMed
-
- Amlacher S, Sarges P, Flemming D, van Noort V, Kunze R, Devos DP, Arumugam M, Bork P, Hurt E. Insight into structure and assembly of the nuclear pore complex by utilizing the genome of a eukaryotic thermophile. Cell. 2011;146:277–289. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

