Safety and efficacy profile of pembrolizumab in solid cancer: pooled reanalysis based on randomized controlled trials
- PMID: 29033546
- PMCID: PMC5628692
- DOI: 10.2147/DDDT.S146286
Safety and efficacy profile of pembrolizumab in solid cancer: pooled reanalysis based on randomized controlled trials
Abstract
Background: The aim of the present review is to systematically evaluate the efficacy and safety of pembrolizumab by analyzing survival outcomes and at the same time, to present evidence for future clinical applications of anti-programmed cell death protein 1 (anti-PD-1) antibodies by analyzing the efficacy and safety of pembrolizumab.
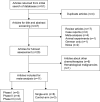
Methods: A comprehensive literature search of PubMed, Medline, and Embase was performed for all relevant clinical trials. In this study, adverse events of any grades and grades ≥3 were summarized and calculated for event rates. For controlled trials, odd ratios (ORs) were calculated to determine the role of pembrolizumab in adverse events. The Kaplan-Meier survival curves were extracted for hazard ratio (HR) calculation and survival outcomes were measured by progression-free survival (PFS).
Results: A total of 3,953 patients were included in safety analyses. The results indicated that the overall incidence of any treatment emergent adverse events was 74.3% (95% confidence interval [CI]: 0.671-0.805). The efficacy analysis involving 915 patients with advanced melanoma suggested that 10 mg/kg of pembrolizumab every 3 weeks could improve patients' PFS (HR =0.73, 95% CI: 0.64-0.83).
Conclusion: Pembrolizumab is a promising therapeutic option that could bring better survival outcomes but, at the same time, leads to higher frequency of some adverse events.
Keywords: efficacy; meta-analysis; pembrolizumab; safety.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures






References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

