Effect of surface organic coatings of cellulose nanocrystals on the viability of mammalian cell lines
- PMID: 29033558
- PMCID: PMC5628661
- DOI: 10.2147/NSA.S145891
Effect of surface organic coatings of cellulose nanocrystals on the viability of mammalian cell lines
Abstract
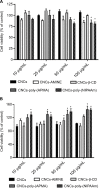
Cellulose nanocrystals (CNCs) have emerged as promising candidates for a number of bio-applications. Surface modification of CNCs continues to gain significant research interest as it imparts new properties to the surface of the nanocrystals for the design of multifunctional CNCs-based materials. A small chemical surface modification can potentially lead to drastic behavioral changes of cell-material interactions thereby affecting the intended bio-application. In this work, unmodified CNCs were covalently decorated with four different organic moieties such as a diaminobutane fragment, a cyclic oligosaccharide (β-cyclodextrin), a thermoresponsive polymer (poly[N-isopropylacrylamide]), and a cationic aminomethacrylamide-based polymer using different synthetic covalent methods. The effect of surface coatings of CNCs and the respective dose-response of the above organic moieties on the cell viability were evaluated on mammalian cell cultures (J774A.1 and MFC-7), using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphe-nyltetrazolium bromide and lactate dehydrogenase assays. Overall, the results indicated that cells exposed to surface-coated CNCs for 24 h did not display major changes in cell viability, membrane permeability as well as cell morphology. However, with longer exposure, all these parameters were somewhat affected, which appears not to be correlated with either anionic or cationic surface coatings of CNCs used in this study.
Keywords: LDH; MTT; cell viability; cellulose nanocrystals; surface coating.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Synthesis and Cytotoxicity Studies of Wood-Based Cationic Cellulose Nanocrystals as Potential Immunomodulators.Nanomaterials (Basel). 2020 Aug 15;10(8):1603. doi: 10.3390/nano10081603. Nanomaterials (Basel). 2020. PMID: 32824129 Free PMC article.
-
Vascular and Blood Compatibility of Engineered Cationic Cellulose Nanocrystals in Cell-Based Assays.Nanomaterials (Basel). 2021 Aug 15;11(8):2072. doi: 10.3390/nano11082072. Nanomaterials (Basel). 2021. PMID: 34443903 Free PMC article.
-
Cationic poly(2-aminoethylmethacrylate) and poly(N-(2-aminoethylmethacrylamide) modified cellulose nanocrystals: synthesis, characterization, and cytotoxicity.Biomacromolecules. 2015 Jan 12;16(1):319-25. doi: 10.1021/bm501516r. Epub 2014 Dec 9. Biomacromolecules. 2015. PMID: 25436513
-
Cellulose nanocrystals: a versatile nanoplatform for emerging biomedical applications.Expert Opin Drug Deliv. 2016 Sep;13(9):1243-56. doi: 10.1080/17425247.2016.1182491. Epub 2016 May 9. Expert Opin Drug Deliv. 2016. PMID: 27110733 Review.
-
State-of-art review on preparation, surface functionalization and biomedical applications of cellulose nanocrystals-based materials.Int J Biol Macromol. 2021 Sep 1;186:591-615. doi: 10.1016/j.ijbiomac.2021.07.066. Epub 2021 Jul 14. Int J Biol Macromol. 2021. PMID: 34271046 Review.
Cited by
-
Combining Cellulose and Cyclodextrins: Fascinating Designs for Materials and Pharmaceutics.Front Chem. 2018 Jul 5;6:271. doi: 10.3389/fchem.2018.00271. eCollection 2018. Front Chem. 2018. PMID: 30027091 Free PMC article. Review.
-
Cellulose Nano-Films as Bio-Interfaces.Front Chem. 2019 Jul 30;7:535. doi: 10.3389/fchem.2019.00535. eCollection 2019. Front Chem. 2019. PMID: 31417896 Free PMC article. Review.
-
Production and Mechanical Characterisation of TEMPO-Oxidised Cellulose Nanofibrils/β-Cyclodextrin Films and Cryogels.Molecules. 2020 May 20;25(10):2381. doi: 10.3390/molecules25102381. Molecules. 2020. PMID: 32443918 Free PMC article.
-
Synthesis, Characterization and Cytotoxicity Studies of Aminated Microcrystalline Cellulose Derivatives against Melanoma and Breast Cancer Cell Lines.Polymers (Basel). 2020 Nov 10;12(11):2634. doi: 10.3390/polym12112634. Polymers (Basel). 2020. PMID: 33182562 Free PMC article.
-
Synthesis and Cytotoxicity Studies of Wood-Based Cationic Cellulose Nanocrystals as Potential Immunomodulators.Nanomaterials (Basel). 2020 Aug 15;10(8):1603. doi: 10.3390/nano10081603. Nanomaterials (Basel). 2020. PMID: 32824129 Free PMC article.
References
-
- Klemm D, Kramer F, Moritz S, et al. Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed Engl. 2011;50(24):5438–5466. - PubMed
-
- Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev. 2011;40(7):3941–3994. - PubMed
-
- Habibi Y, Lucia LA, Rojas OJ. Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev. 2010;110(6):3479–3500. - PubMed
-
- Eichhorn SJ. Cellulose nanowhiskers: promising materials for advanced applications. Soft Matter. 2011;7(2):303–315.
-
- Peng BL, Dhar N, Liu HL, Tam KC. Chemistry and applications of nanocrystalline cellulose and its derivatives: a nanotechnology perspective. Can J Chem Eng. 2011;89(5):1191–1206.
LinkOut - more resources
Full Text Sources
Other Literature Sources

