Wnt5a Promotes Cortical Neuron Survival by Inhibiting Cell-Cycle Activation
- PMID: 29033786
- PMCID: PMC5626855
- DOI: 10.3389/fncel.2017.00281
Wnt5a Promotes Cortical Neuron Survival by Inhibiting Cell-Cycle Activation
Abstract
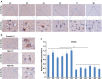
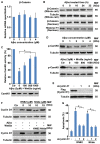
β-Amyloid protein (Aβ) is thought to cause neuronal loss in Alzheimer's disease (AD). Aβ treatment promotes the re-activation of a mitotic cycle and induces rapid apoptotic death of neurons. However, the signaling pathways mediating cell-cycle activation during neuron apoptosis have not been determined. We find that Wnt5a acts as a mediator of cortical neuron survival, and Aβ42 promotes cortical neuron apoptosis by downregulating the expression of Wnt5a. Cell-cycle activation is mediated by the reduced inhibitory effect of Wnt5a in Aβ42 treated cortical neurons. Furthermore, Wnt5a signals through the non-canonical Wnt/Ca2+ pathway to suppress cyclin D1 expression and negatively regulate neuronal cell-cycle activation in a cell-autonomous manner. Together, aberrant downregulation of Wnt5a signaling is a crucial step during Aβ42 induced cortical neuron apoptosis and might contribute to AD-related neurodegeneration.
Keywords: Alzheimer’s disease; Cyclin D1; Wnt5a; apoptosis; cell-cycle activation; cortical neuron; β-Amyloid protein.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

