Activation of Casein Kinase II by Gallic Acid Induces BIK-BAX/BAK-Mediated ER Ca++-ROS-Dependent Apoptosis of Human Oral Cancer Cells
- PMID: 29033852
- PMCID: PMC5627504
- DOI: 10.3389/fphys.2017.00761
Activation of Casein Kinase II by Gallic Acid Induces BIK-BAX/BAK-Mediated ER Ca++-ROS-Dependent Apoptosis of Human Oral Cancer Cells
Abstract
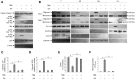
Induction of the generation of endoplasmic reticulum (ER) calcium (Ca++)-mediated reactive oxygen species (ROS) by gallic acid (GA) has been implicated in the mitochondrial apoptotic death of human oral cancer (OC) cells, but the molecular mechanism by which GA causes ER Ca++ release of OC cells to undergo cell death remains unclear. Here, we report that GA-induced phosphorylation of B-cell lymphoma 2 (BCL-2)-interacting killer (BIK) (threonine (Thr) 33/Serine (Ser) 35) and p53 (Ser 15 and Ser 392), Bcl-2-associated x protein (BAX)/BCL-2 antagonist killer 1 (BAK) oligomerization on the ER and mitochondria, rising of cytosolic Ca++ and ROS, cytochrome c (Cyt c) release from the mitochondria, Ψm loss, and apoptosis were suppressed in cells co-treated with a specific inhibitor of casein kinase II (CK II) (4,5,6,7-tetrabromobenzotriazole). Small interfering RNA (siRNA)-mediated suppression of BIK inhibited GA-induced oligomeric complex of BAX/BAK in the ER and mitochondria, increase of cytosolic Ca++ and ROS, and apoptosis, but did not attenuate the increase in the level of Ser 15-phosphated p53 induced by GA. Blockade of p53 expression by short hairpin RNA suppressed BAX/BAK oligomerization and ER Ca++-ROS-associated apoptosis induced by GA but did not affect GA-induced phospho-BIK (Thr 33/Ser 35) levels. Induction of mitochondrial Cyt c release and ROS generation, increased cytosolic Ca++ level, and apoptosis by GA was attenuated by expression of the BAX or BAK siRNA. Over-expression of BCL-2 (but not BCL-XL) inhibited formation of ER oligomeric BAX/BAK by GA. Our results demonstrated that activation of the CK II by GA is required for the BIK-mediated ROS-dependent apoptotic activity of ER-associated BAX/BAK.
Keywords: BAX/BAK; BIK; ER Ca++; ROS; casein kinase II; gallic acid.
Figures




References
-
- Abdelwahed A., Bouhlel I., Skandrani I., Valenti K., Kadri M., Guiraud P., et al. . (2007). Study of antimutagenic and antioxidant activities of gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus. Confirmation by microarray expression profiling. Chem. Biol. Interact. 165, 1–13. 10.1016/j.cbi.2006.10.003 - DOI - PubMed
-
- Agarwal C., Tyagi A., Agarwal R. (2006). Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells. Mol. Cancer Ther. 5, 3294–3302. 10.1158/1535-7163.MCT-06-0483 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

