Spermine Regulates Pollen Tube Growth by Modulating Ca2+-Dependent Actin Organization and Cell Wall Structure
- PMID: 29033970
- PMCID: PMC5627395
- DOI: 10.3389/fpls.2017.01701
Spermine Regulates Pollen Tube Growth by Modulating Ca2+-Dependent Actin Organization and Cell Wall Structure
Abstract
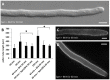
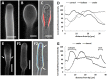
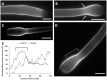
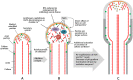
Proper growth of the pollen tube depends on an elaborate mechanism that integrates several molecular and cytological sub-processes and ensures a cell shape adapted to the transport of gametes. This growth mechanism is controlled by several molecules among which cytoplasmic and apoplastic polyamines. Spermine (Spm) has been correlated with various physiological processes in pollen, including structuring of the cell wall and modulation of protein (mainly cytoskeletal) assembly. In this work, the effects of Spm on the growth of pear pollen tubes were analyzed. When exogenous Spm (100 μM) was supplied to germinating pollen, it temporarily blocked tube growth, followed by the induction of apical swelling. This reshaping of the pollen tube was maintained also after growth recovery, leading to a 30-40% increase of tube diameter. Apical swelling was also accompanied by a transient increase in cytosolic calcium concentration and alteration of pH values, which were the likely cause for major reorganization of actin filaments and cytoplasmic organelle movement. Morphological alterations of the apical and subapical region also involved changes in the deposition of pectin, cellulose, and callose in the cell wall. Thus, results point to the involvement of Spm in cell wall construction as well as cytoskeleton organization during pear pollen tube growth.
Keywords: actin dynamics; callose; cell wall; cellulose; cytosolic calcium; pectins; pollen; spermine.
Figures









References
-
- Alburquerque N., Burgos L., Sedgley M., Egea J. (2004). Contributing to the knowledge of the fertilization process in four apricot cultivars. Sci. Hortic. 102, 387–396. 10.1016/j.scienta.2004.02.003 - DOI
-
- Alburquerque N., Egea J., Burgos L., Martínez-Romero D., Valero D., Serrano M. (2006). The influence of polyamines on apricot ovary development and fruit set. Ann. Appl. Biol. 149, 27–33. 10.1111/j.1744-7348.2006.00067.x - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

