Genomic insights into head and neck cancer
- PMID: 29034103
- PMCID: PMC5638139
- DOI: 10.1186/s41199-016-0003-z
Genomic insights into head and neck cancer
Abstract
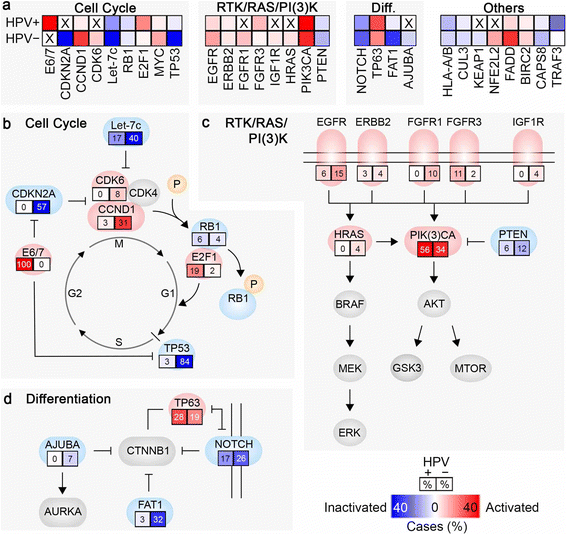
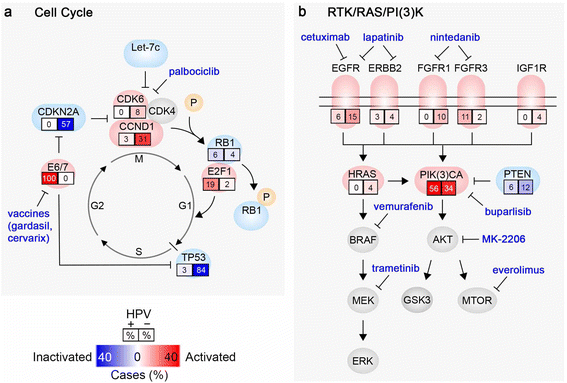
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide and is frequently impervious to curative treatment efforts. Similar to other cancers associated with prolonged exposure to carcinogens, HNSCCs often have a high burden of mutations, contributing to substantial inter- and intra-tumor heterogeneity. The heterogeneity of this malignancy is further increased by the rising rate of human papillomavirus (HPV)-associated (HPV+) HNSCC, which defines an etiological subtype significantly different from the more common tobacco and alcohol associated HPV-negative (HPV-) HNSCC. Since 2011, application of large scale genome sequencing projects by The Cancer Genome Atlas (TCGA) network and other groups have established extensive datasets to characterize HPV- and HPV+ HNSCC, providing a foundation for advanced molecular diagnoses, identification of potential biomarkers, and therapeutic insights. Some genomic lesions are now appreciated as widely dispersed. For example, HPV- HNSCC characteristically inactivates the cell cycle suppressors TP53 (p53) and CDKN2A (p16), and often amplifies CCND1 (cyclin D), which phosphorylates RB1 to promote cell cycle progression from G1 to S. By contrast, HPV+ HNSCC expresses viral oncogenes E6 and E7, which inhibit TP53 and RB1, and activates the cell cycle regulator E2F1. Frequent activating mutations in PIK3CA and inactivating mutations in NOTCH1 are seen in both subtypes of HNSCC, emphasizing the importance of these pathways. Studies of large patient cohorts have also begun to identify less common genetic alterations, predominantly found in HPV- tumors, which suggest new mechanisms relevant to disease pathogenesis. Targets of these alterations including AJUBA and FAT1, both involved in the regulation of NOTCH/CTNNB1 signaling. Genes involved in oxidative stress, particularly CUL3, KEAP1 and NFE2L2, strongly associated with smoking, have also been identified, and are less well understood mechanistically. Application of sophisticated data-mining approaches, integrating genomic information with profiles of tumor methylation and gene expression, have helped to further yield insights, and in some cases suggest additional approaches to stratify patients for clinical treatment. We here discuss some recent insights built on TCGA and other genomic foundations.
Keywords: Cancer therapy; Cell cycle; Genomics; HPV; Head and neck cancer; Personalized medicine; TCGA; Tumor heterogeneity.
Conflict of interest statement
Competing interests The authors declare that they have no competing interests.
Figures
References
-
- Burtness B, Golemis EA. Overview: the pathobiology of head and neck cancer. In: Burtness B, Golemis EA, editors. Molecular determinants of head and neck cancer. 1. New York: Springer New York; 2014. pp. 1–5.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
