Misoprostol Inhibits Lipopolysaccharide-Induced Pro-inflammatory Cytokine Production by Equine Leukocytes
- PMID: 29034249
- PMCID: PMC5624997
- DOI: 10.3389/fvets.2017.00160
Misoprostol Inhibits Lipopolysaccharide-Induced Pro-inflammatory Cytokine Production by Equine Leukocytes
Abstract
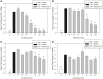
Pro-inflammatory cytokines including tumor necrosis factor α (TNFα), IL-1β, IL-6, and IL-8 are potent immune mediators that exacerbate multiple equine diseases such as sepsis and laminitis. Unfortunately, safe and effective cytokine-targeting therapies are lacking in horses; therefore, novel mechanisms of inhibiting cytokine production are critically needed. One potential mechanism for inhibiting cytokine synthesis is elevation of intracellular cyclic AMP (cAMP). In human leukocytes, intracellular cAMP production is induced by activation of E-prostanoid (EP) receptors 2 and 4. These receptors can be targeted by the EP2/4 agonist and prostaglandin E1 analog, misoprostol. Misoprostol is currently used as a gastroprotectant in horses but has not been evaluated as a cytokine-targeting therapeutic. Thus, we hypothesized that misoprostol treatment would inhibit pro-inflammatory cytokine production by lipopolysaccharide (LPS)-stimulated equine leukocytes in an in vitro inflammation model. To test this hypothesis, equine leukocyte-rich plasma (LRP) was collected from 12 healthy adult horses and used to model LPS-mediated inflammatory signaling. LRP was treated with varying concentrations of misoprostol either before (pretreated) or following (posttreated) LPS stimulation. LRP supernatants were assayed for 23 cytokines using an equine-specific multiplex bead immunoassay. Leukocytes were isolated from LRP, and leukocyte mRNA levels of four important cytokines were evaluated via RT-PCR. Statistical differences between treatments were determined using one-way RM ANOVA (Holm-Sidak post hoc testing) or Friedman's RM ANOVA on Ranks (SNK post hoc testing), where appropriate (p < 0.05, n = 3-6 horses). These studies revealed that misoprostol pre- and posttreatment inhibited LPS-induced TNFα and IL-6 protein production in equine leukocytes but had no effect on IL-8 protein. Interestingly, misoprostol pretreatment enhanced IL-1β protein synthesis following 6 h of LPS stimulation, while misoprostol posttreatment inhibited IL-1β protein production after 24 h of LPS stimulation. At the mRNA level, misoprostol pre- and posttreatment inhibited LPS-induced TNFα, IL-1β, and IL-6 mRNA production but did not affect IL-8 mRNA. These results indicate that misoprostol exerts anti-inflammatory effects on equine leukocytes when applied before or after a pro-inflammatory stimulus. However, the effects we observed were cytokine-specific and sometimes differed at the mRNA and protein levels. Further studies are warranted to establish the inhibitory effects of misoprostol on equine cytokine production in vivo.
Keywords: anti-inflammatory; chemokine; horse; inflammation; leukocyte; non-steroidal anti-inflammatory drug.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

