Emergence of β-Band Oscillations in the Aged Rat Amygdala during Discrimination Learning and Decision Making Tasks
- PMID: 29034315
- PMCID: PMC5629614
- DOI: 10.1523/ENEURO.0245-17.2017
Emergence of β-Band Oscillations in the Aged Rat Amygdala during Discrimination Learning and Decision Making Tasks
Abstract
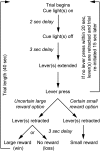
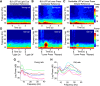
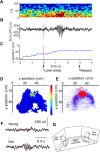
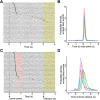
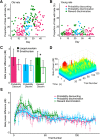
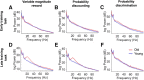
Older adults tend to use strategies that differ from those used by young adults to solve decision-making tasks. MRI experiments suggest that altered strategy use during aging can be accompanied by a change in extent of activation of a given brain region, inter-hemispheric bilateralization or added brain structures. It has been suggested that these changes reflect compensation for less effective networks to enable optimal performance. One way that communication can be influenced within and between brain networks is through oscillatory events that help structure and synchronize incoming and outgoing information. It is unknown how aging impacts local oscillatory activity within the basolateral complex of the amygdala (BLA). The present study recorded local field potentials (LFPs) and single units in old and young rats during the performance of tasks that involve discrimination learning and probabilistic decision making. We found task- and age-specific increases in power selectively within the β range (15-30 Hz). The increased β power occurred after lever presses, as old animals reached the goal location. Periods of high-power β developed over training days in the aged rats, and was greatest in early trials of a session. β Power was also greater after pressing for the large reward option. These data suggest that aging of BLA networks results in strengthened synchrony of β oscillations when older animals are learning or deciding between rewards of different size. Whether this increased synchrony reflects the neural basis of a compensatory strategy change of old animals in reward-based decision-making tasks, remains to be verified.
Keywords: basolateral complex of the amygdala; probability discounting; probability discrimination; reward expectation; reward magnitude discrimination.
Figures



Similar articles
-
Optogenetic Dissection of Temporal Dynamics of Amygdala-Striatal Interplay during Risk/Reward Decision Making.eNeuro. 2018 Dec 10;5(6):ENEURO.0422-18.2018. doi: 10.1523/ENEURO.0422-18.2018. eCollection 2018 Nov-Dec. eNeuro. 2018. PMID: 30627636 Free PMC article.
-
Fundamental contribution by the basolateral amygdala to different forms of decision making.J Neurosci. 2009 Apr 22;29(16):5251-9. doi: 10.1523/JNEUROSCI.0315-09.2009. J Neurosci. 2009. PMID: 19386921 Free PMC article.
-
Basolateral amygdala - nucleus accumbens circuitry regulates optimal cue-guided risk/reward decision making.Prog Neuropsychopharmacol Biol Psychiatry. 2020 Mar 2;98:109830. doi: 10.1016/j.pnpbp.2019.109830. Epub 2019 Dec 4. Prog Neuropsychopharmacol Biol Psychiatry. 2020. PMID: 31811876
-
Dissociable roles for the basolateral amygdala and orbitofrontal cortex in decision-making under risk of punishment.J Neurosci. 2015 Jan 28;35(4):1368-79. doi: 10.1523/JNEUROSCI.3586-14.2015. J Neurosci. 2015. PMID: 25632115 Free PMC article.
-
Functional disconnection of the orbitofrontal cortex and basolateral amygdala impairs acquisition of a rat gambling task and disrupts animals' ability to alter decision-making behavior after reinforcer devaluation.J Neurosci. 2013 Apr 10;33(15):6434-43. doi: 10.1523/JNEUROSCI.3971-12.2013. J Neurosci. 2013. PMID: 23575841 Free PMC article.
Cited by
-
Dynamic switching of neural oscillations in the prefrontal-amygdala circuit for naturalistic freeze-or-flight.Proc Natl Acad Sci U S A. 2023 Sep 12;120(37):e2308762120. doi: 10.1073/pnas.2308762120. Epub 2023 Sep 5. Proc Natl Acad Sci U S A. 2023. PMID: 37669394 Free PMC article.
-
Behavioral Interventions Can Improve Brain Injury-Induced Deficits in Behavioral Flexibility and Impulsivity Linked to Impaired Reward-Feedback Beta Oscillations.J Neurotrauma. 2024 Jul;41(13-14):e1721-e1737. doi: 10.1089/neu.2023.0448. Epub 2024 May 3. J Neurotrauma. 2024. PMID: 38450560 Free PMC article.
-
Beta-band power modulation in the human amygdala during a Direct Reach arm reaching task.Neurosci Res. 2025 Jul;216:104906. doi: 10.1016/j.neures.2025.05.001. Epub 2025 May 11. Neurosci Res. 2025. PMID: 40360082 Free PMC article.
-
Cortico-striatal beta oscillations as a reward-related signal.Cogn Affect Behav Neurosci. 2024 Oct;24(5):839-859. doi: 10.3758/s13415-024-01208-6. Epub 2024 Aug 15. Cogn Affect Behav Neurosci. 2024. PMID: 39147929 Free PMC article.
-
Person-Based Similarity Index for Cognition and Its Neural Correlates in Late Adulthood: Implications for Cognitive Reserve.Cereb Cortex. 2022 Jan 10;32(2):397-407. doi: 10.1093/cercor/bhab215. Cereb Cortex. 2022. PMID: 34255824 Free PMC article.
References
-
- Bates D, Maechler M, Bolker B, Walker S (2015) Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software, 67:1–48. 10.18637/jss.v067.i01 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
