Well-defined nickel and palladium precatalysts for cross-coupling
- PMID: 29034333
- PMCID: PMC5637737
- DOI: 10.1038/s41570-017-0025
Well-defined nickel and palladium precatalysts for cross-coupling
Abstract
Transition metal-catalysed cross-coupling is one of the most powerful synthetic methods and has led to vast improvements in the synthesis of pharmaceuticals, agrochemicals and precursors for materials chemistry. A major advance in cross-coupling over the past 20 years is the utilization of well-defined, bench-stable Pd and Ni precatalysts that do not require the addition of free ancillary ligand, which can hinder catalysis by occupying open coordination sites on the metal. The development of precatalysts has resulted in new reactions and expanded substrate scopes, enabling transformations under milder conditions and with lower catalyst loadings. This Review highlights recent advances in the development of Pd and Ni precatalysts for cross-coupling, and provides a critical comparison between the state of the art in Pd- and Ni-based systems.
Conflict of interest statement
Competing interest statement The authors declare no competing interests.
Figures




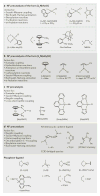
References
-
- Nicolaou KC, Bulger PG, Sarlah D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew Chem Int Ed. 2005;44:4442–4489. - PubMed
-
- Corbet JP, Mignani G. Selected patented cross-coupling reaction technologies. Chem Rev. 2006;106:2651–2710. - PubMed
-
- Magano J, Dunetz JR. Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem Rev. 2011;111:2177–2250. - PubMed
-
- Colacot TJ, editor. New Trends in Cross-Coupling: Theory and Applications. Royal Society of Chemistry; 2015.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

