Prefrontal Connectivity and Glutamate Transmission: Relevance to Depression Pathophysiology and Ketamine Treatment
- PMID: 29034354
- PMCID: PMC5635826
- DOI: 10.1016/j.bpsc.2017.04.006
Prefrontal Connectivity and Glutamate Transmission: Relevance to Depression Pathophysiology and Ketamine Treatment
Abstract
Background: Prefrontal global brain connectivity with global signal regression (GBCr) was proposed as a robust biomarker of depression, and was associated with ketamine's mechanism of action. Here, we investigated prefrontal GBCr in treatment-resistant depression (TRD) at baseline and following treatment. Then, we conducted a set of pharmacological challenges in healthy subjects to investigate the glutamate neurotransmission correlates of GBCr.
Methods: In study A, we used functional magnetic resonance imaging (fMRI) to compare GBCr between 22 TRD and 29 healthy control. Then, we examined the effects of ketamine and midazolam on GBCr in TRD patients 24h post-treatment. In study B, we acquired repeated fMRI in 18 healthy subjects to determine the effects of lamotrigine (a glutamate release inhibitor), ketamine, and lamotrigine-by-ketamine interaction.
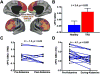
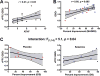
Results: In study A, TRD patients showed significant reduction in dorsomedial and dorsolateral prefrontal GBCr compared to healthy control. In TRD patients, GBCr in the altered clusters significantly increased 24h following ketamine (effect size = 1.0 [0.3 1.8]), but not midazolam (effect size = 0.5 [-0.6 1.3]). In study B, oral lamotrigine reduced GBCr 2h post-administration, while ketamine increased medial prefrontal GBCr during infusion. Lamotrigine significantly reduced the ketamine-induced GBCr surge. Exploratory analyses showed elevated ventral prefrontal GBCr in TRD and significant reduction of ventral prefrontal GBCr during ketamine infusion in healthy subjects.
Conclusions: This study provides first replication of the ability of ketamine to normalize depression-related prefrontal dysconnectivity. It also provides indirect evidence that these effects may be triggered by the capacity of ketamine to enhance glutamate neurotransmission.
Keywords: Treatment resistant depression; functional MRI; global brain connectivity; glutamate; ketamine; rapid acting antidepressants.
Conflict of interest statement
Conflict of Interests CGA has served as a consultant and/or on advisory boards for Genentech and Janssen, and on editorial for Sage Publications, Inc. JHK is a consultant for AbbVie, Inc., Amgen, Astellas Pharma Global Development, Inc., AstraZeneca Pharmaceuticals, Biomedisyn Corporation, Bristol-Myers Squibb, Eli Lilly and Company, Euthymics Bioscience, Inc., Neurovance, Inc., FORUM Pharmaceuticals, Janssen Research & Development, Lundbeck Research USA, Novartis Pharma AG, Otsuka America Pharmaceutical, Inc., Sage Therapeutics, Inc., Sunovion Pharmaceuticals, Inc., and Takeda Industries; is on the Scientific Advisory Board for Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Inc., Naurex, Inc., and Pfizer; is a stockholder in Biohaven Medical Sciences; holds stock options in Mnemosyne Pharmaceuticals, Inc.; holds patents for Dopamine and Noradrenergic Reuptake Inhibitors in Treatment of Schizophrenia, U.S. Patent No. 5,447,948 (issued Sep 5, 1995), and Glutamate Modulating Agents in the Treatment of Mental Disorders, U.S. Patent No. 8,778,979 (issued Jul 15, 2014); and filed a patent for Intranasal Administration of Ketamine to Treat Depression. U.S. Application No. 14/197,767 (filed on Mar 5, 2014); U.S. application or Patent Cooperation Treaty international application No. 14/306,382 (filed on Jun 17, 2014). SJM: In the past 12 months, Dr. Mathew has received consulting fees from Acadia, Alkermes, Allergan, Cerecor, Otsuka, and Valeant, and serves on an Advisory Board for VistaGen Therapeutics. He has received research support from Janssen Research & Development. DHM is a consultant for Boehringer Ingelheim, Takeda, Alkermes, and Upsher-Smith. All other authors report no competing interests.
Figures
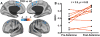
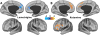

Comment in
-
Using Neuroimaging to Decipher the Mechanism of Action of Ketamine: A Pathway to Novel Therapeutics?Biol Psychiatry Cogn Neurosci Neuroimaging. 2017 Oct;2(7):549-551. doi: 10.1016/j.bpsc.2017.08.006. Epub 2017 Oct 5. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017. PMID: 29560906 Free PMC article. No abstract available.
References
-
- Murrough JW, Abdallah CG, Mathew S. Targeting glutamate signaling in depression: progress and prospects. Nature Reviews: Drug Discovery. 2017 In Press. - PubMed
-
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. - PubMed
