The Definition, Rationale, and Effects of Thresholding in OCT Angiography
- PMID: 29034359
- PMCID: PMC5640169
- DOI: 10.1016/j.oret.2017.01.019
The Definition, Rationale, and Effects of Thresholding in OCT Angiography
Abstract
Purpose: To examine the definition, rationale, and effects of thresholding in OCT angiography (OCTA).
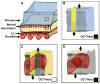
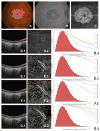
Design: A theoretical description of OCTA thresholding in combination with qualitative and quantitative analysis of the effects of OCTA thresholding in eyes from a retrospective case series.
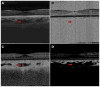
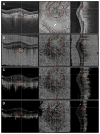
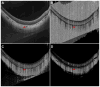
Participants: Four eyes were qualitatively examined: 1 from a 27-year-old control, 1 from a 78-year-old exudative age-related macular degeneration (AMD) patient, 1 from a 58-year-old myopic patient, and 1 from a 77-year-old nonexudative AMD patient with geographic atrophy (GA). One eye from a 75-year-old nonexudative AMD patient with GA was quantitatively analyzed.
Main outcome measures: A theoretical thresholding model and a qualitative and quantitative description of the dependency of OCTA on thresholding level.
Results: Due to the presence of system noise, OCTA thresholding is a necessary step in forming OCTA images; however, thresholding can complicate the relationship between blood flow and OCTA signal.
Conclusions: Thresholding in OCTA can cause significant artifacts, which should be considered when interpreting and quantifying OCTA images.
Conflict of interest statement
Conflicts of Interest: J.S.D.: Consultant and research support — Carl Zeiss Meditec, OptoVue, and Topcon Medical Systems Inc; stock — Hemera Biosciences Inc, EyeNetra, and Ophthotech Corp. N.K.W.: Consultant — Iconic Therapeutics; speaker’s bureau —ThromboGenics; research support — Carl Zeiss Meditec, Inc. J.G.F.: Royalties — intellectual property owned by the Massachusetts Institute of Technology and licensed to Carl Zeiss Meditec Inc, Optovue Inc; stock options — Optovue Inc. There are no conflicting relationships for any other author.
Figures



References
-
- Makita S, Hong Y, Yamanari M, et al. Optical coherence angiography. Opt Express. 2006;14(17):7821–7840. - PubMed
-
- Mahmud MS, Cadotte DW, Vuong B, et al. Review of speckle and phase variance optical coherence tomography to visualize microvascular networks. J Biomed Opt. 2013;18(5):50901. - PubMed
-
- Mariampillai A, Standish BA, Moriyama EH, et al. Speckle variance detection of microvasculature using swept-source optical coherence tomography. Opt Lett. 2008;33(13):1530–1532. - PubMed
-
- An L, Wang RK. In vivo volumetric imaging of vascular perfusion within human retina and choroids with optical micro-angiography. Opt Express. 2008;16(15):11438–11452. - PubMed
-
- Makita S, Jaillon F, Yamanari M, et al. Comprehensive in vivo micro-vascular imaging of the human eye by dual-beam-scan Doppler optical coherence angiography. Opt Express. 2011;19(2):1271–1283. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
