Inhaled versus systemic corticosteroids for the treatment of bronchopulmonary dysplasia in ventilated very low birth weight preterm infants
- PMID: 29035425
- PMCID: PMC6485655
- DOI: 10.1002/14651858.CD002057.pub4
Inhaled versus systemic corticosteroids for the treatment of bronchopulmonary dysplasia in ventilated very low birth weight preterm infants
Abstract
Background: This is an update of a review published in 2012. A related review "Inhaled versus systemic corticosteroids for preventing bronchopulmonary dysplasia in ventilated very low birth weight preterm neonates" has been updated as well. Bronchopulmonary dysplasia (BPD) is a serious and common problem among very low birth weight infants, despite the use of antenatal steroids and postnatal surfactant therapy to decrease the incidence and severity of respiratory distress syndrome. Due to their anti-inflammatory properties, corticosteroids have been widely used to treat or prevent BPD. However, the use of systemic steroids has been associated with serious short- and long-term adverse effects. Administration of corticosteroids topically through the respiratory tract may result in beneficial effects on the pulmonary system with fewer undesirable systemic side effects.
Objectives: To compare the effectiveness of inhaled versus systemic corticosteroids administered to ventilator-dependent preterm neonates with birth weight ≤ 1500 g or gestational age ≤ 32 weeks after 7 days of life on the incidence of death or BPD at 36 weeks' postmenstrual age.
Search methods: We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 1), MEDLINE via PubMed (1966 to 23 February 2017), Embase (1980 to 23 February 2017), and CINAHL (1982 to 23 February 2017). We also searched clinical trials registers, conference proceedings and the reference lists of retrieved articles for randomised controlled trials and quasi-randomised trials.
Selection criteria: Randomised or quasi-randomised controlled trials comparing inhaled versus systemic corticosteroid therapy (irrespective of dose and duration) starting after the first week of life in ventilator-dependent very low birth weight infants.
Data collection and analysis: We used standard methodological procedures expected by the Cochrane Collaboration.
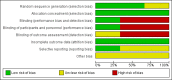
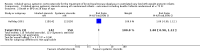
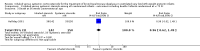
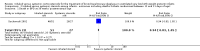
Main results: We included three trials that involved a total of 431 participants which compared inhaled versus systemic corticosteroids to treat BPD. No new trials were included for the 2017 update.Although one study randomised infants at < 72 hours (N = 292), treatment started when infants were aged > 15 days. In this larger study, deaths were included from the point of randomisation and before treatment started. Two studies (N = 139) randomised and started treatment at 12 to 21 days.Two trials reported non-significant differences between groups for the primary outcome: incidence of death or BPD at 36 weeks' postmenstrual age among all randomised infants. Estimates for the largest trial were Relative risk (RR) 1.04 (95% Confidence interval (CI) 0.86 to 1.26), Risk difference (RD) 0.03 (95% CI -0.09 to 0.15); (moderate-quality evidence). Estimates for the other trial reporting the primary outcome were RR 0.94 (95% CI 0.83 to 1.05), RD -0.06 (95% CI -0.17 to 0.05); (low-quality evidence).Secondary outcomes that included data from all three trials showed no significant differences in the duration of mechanical ventilation or supplemental oxygen, length of hospital stay, or the incidence of hyperglycaemia, hypertension, necrotising enterocolitis, gastrointestinal bleed, retinopathy of prematurity or culture-proven sepsis moderate- to low-quality evidence).In a subset of 75 surviving infants who were enrolled from the United Kingdom and Ireland, there were no significant differences in developmental outcomes at seven years of age between groups (moderate-quality evidence). One study received grant support and the industry provided aerochambers and metered dose inhalers of budesonide and placebo for the same study. No conflict of interest was identified.
Authors' conclusions: We found no evidence that inhaled corticosteroids confer net advantages over systemic corticosteroids in the management of ventilator-dependent preterm infants. There was no evidence of difference in effectiveness or adverse event profiles for inhaled versus systemic steroids.A better delivery system guaranteeing selective delivery of inhaled steroids to the alveoli might result in beneficial clinical effects without increasing adverse events.To resolve this issue, studies are needed to identify the risk/benefit ratio of different delivery techniques and dosing schedules for administration of these medications. The long-term effects of inhaled steroids, with particular attention to neurodevelopmental outcomes, should be addressed in future studies.
Conflict of interest statement
Sachin S Shah, no conflict of interest to declare.
Arne Ohlsson, no conflict of interest to declare.
Henry L Halliday, is the author of an included trial. He did not assess the quality of his own trial.
Vibhuti S Shah, no conflict of interest to declare.
Figures
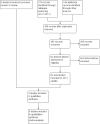




























Update of
-
Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants.Cochrane Database Syst Rev. 2012 May 16;(5):CD002057. doi: 10.1002/14651858.CD002057.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Oct 16;10:CD002057. doi: 10.1002/14651858.CD002057.pub4. PMID: 22592682 Updated.
References
References to studies included in this review
Halliday 2001 {published data only}
-
- Halliday HL, Patterson CC, Halahakoon CW, European Multicenter Steroid Study Group. A multicenter, randomized open study of early corticosteroid treatment (OSECT) in preterm infants with respiratory illness: comparison of early and late treatment and of dexamethasone and inhaled budesonide. Pediatrics 2001;107(2):232‐40. - PubMed
-
- Wilson TT. Long Term Neurodevelopmental and Psychosocial Outcomes Following Premature Birth: Has Postnatal Corticosteroid Treatment Been an Over‐looked Factor? [PhD thesis]. Belfast (UK): Queen’s University Belfast, 2005.
-
- Wilson TT, Waters L, Patterson CC, McCusker CG, Rooney NM, Marlow N, et al. Neurodevelopmental and respiratory follow‐up results at 7 years for children from the United Kingdom and Ireland enrolled in a randomized trial of early and late postnatal corticosteroid treatment, systemic and inhaled (the Open Study of Early Corticosteroid Treatment). Pediatrics 2006;117(6):2196‐205. - PubMed
Rozycki 2003 {published data only}
-
- Rozycki HJ, Byron PR, Elliott GR, Carroll T, Gutcher GR. Randomized controlled trial of three different doses of aerosol beclomethasone versus systemic dexamethasone to promote extubation in ventilated premature infants. Pediatric Pulmonology 2003;35(5):375‐83. - PubMed
Suchomski 2002 {published data only}
-
- Suchomski SJ, Cummings JJ. A randomised trial of inhaled versus intravenous steroids in ventilator dependent preterm infants. Journal of Perinatology 2002;22(3):196‐203. - PubMed
References to studies excluded from this review
Dimitriou 1997 {published data only}
-
- Dimitriou G, Greenhough A, Giffin FJ, Kavadia V. Inhaled versus systemic steroids in chronic oxygen dependency of preterm infants. European Journal of Pediatrics 1997;156(1):51‐5. - PubMed
Additional references
AAP & CPS 2002
-
- American Academy of Pediatrics, Committee on Fetus and Newborn, Canadian Paediatric Society, Fetus and Newborn Committee. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics 2002;109(2):330–8. - PubMed
Arnon 1992
-
- Arnon S, Grigg J, Nikander K, Silverman M. Delivery of micronized budesonide suspension by metered dose inhaler and jet nebulizer into a neonatal ventilator circuit. Pediatric Pulmonology 1992;13(3):172‐5. - PubMed
Avery 1985
-
- Avery GB, Fletcher AB, Kaplan M, Brudno DS. Controlled trial of dexamethasone in respirator‐dependent infants with bronchopulmonary dysplasia. Pediatrics 1985;75(1):106‐11. - PubMed
Bahadue 2012
Bassler 2015
-
- Bassler D, Plavka R, Shinvell ES, Hallman M, Jarreau PH, Carnielli V, et al. Early inhaled budesonide for the prevention of bronchopulmonary dysplasia. New England Journal of Medicine 2015;573(16):1497‐506. - PubMed
Bell 1978
Bhuta 1998
CDTG 1991
-
- Collaborative Dexamethasone Trial Group. Dexamethasone therapy in neonatal chronic lung disease: an international placebo controlled trial. Pediatrics 1991;88(3):421‐7. - PubMed
Cummings 1989
-
- Cummings JJ, D'Eugenio DB, Gross SJ. A controlled trial of dexamethasone in preterm infants at high risk for bronchopulmonary dysplasia. New England Journal of Medicine 1989;320(23):1505‐10. - PubMed
Davis 2001
Doyle 2014
Doyle 2014a
Doyle 2017
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 23 February 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Grigg 1992
Gupta 2000
-
- Gupta GK, Cole CH, Abbasi S, Demissie S, Nijnimbam C, Nielsen HC. Effects of early inhaled beclomethasone therapy on tracheal aspirate inflammatory mediators IL8 and IL‐Ira in ventilated preterm infants at risk for bronchopulmonary dysplasia. Pediatric Pulmonology 2000;30(4):275‐81. - PubMed
Halliday 1999
-
- Halliday HL. Clinical trials of postnatal steroids: inhaled and systemic. Biology of the Neonate 1999;76(Suppl 1):29‐40. - PubMed
Halliday 2001a
-
- Halliday HL. Guidelines on neonatal steroids. Prenatal and Neonatal Medicine 2001;6(6):371‐3.
Harkavy 1989
-
- Harkavy KL, Scanlon JW, Chowdhry PK, Grylack LJ. Dexamethasone therapy for chronic lung disease in ventilator‐and‐oxygen‐ dependent infants: a controlled trial. Journal of Pediatrics 1989;115(6):979‐83. - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Cochrane Collaboration.
Ibrahim 2011
ICROP 1984
-
- The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Archives of Ophthalmology 1984;102:1130‐4. - PubMed
Jefferies 2012
Johnson 1979
-
- Johnson JW, Mitzner W, London WT, Palmer AE, Scott R. Betamethasone and the rhesus fetus: multisystemic effects. American Journal of Obstetrics and Gynecology 1979;133(6):677‐84. - PubMed
Kazzi 1990
-
- Kazzi NJ, Brans YW, Poland RL. Dexamethasone effects on the hospital course of infants with bronchopulmonary dysplasia who are dependent on artificial ventilation. Pediatrics 1990;86(5):722‐7. - PubMed
Kelly 2017
-
- Kelly EN, Shah VS, Levenbach J, Vincer M, DaSilva O, Shah PS, Canadian Neonatal Network and Canadian Neonatal Follow‐Up Network Investigators. Inhaled and systemic steroid exposure and neurodevelopmental outcome of preterm neonates. Journal of Maternal‐fetal & Neonatal Medicine 2017 July 16 [Epub ahead of print]. [DOI: 10.1080/14767058.2017.1350644; PUBMED: 28714339] - DOI - PubMed
Lee 2000
-
- Lee SK, McMillan DD, Ohlsson A, Pendray M, Synnes A, Whyte R, et al. Variations in practice and outcomes in the Canadian NICU Network: 1996‐1997. Pediatrics 2000;106(5):1070‐9. - PubMed
Lemons 2001
-
- Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, et al. Very low birth weight outcomes of National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics 2001;107(1):E1. - PubMed
Nelin 2017
Ng 1993
O'Callaghan 1992
O'Shea 1999
-
- O'Shea TM, Kothadia JM, Klinepeter KL, Goldstein DJ, Jackson BG, Weaver RG 3rd, et al. Randomized placebo‐controlled trial of a 42 day tapering course of dexamethasone to reduce the duration of ventilatory dependency in very low birth weight infants: outcomes of study participants at 1‐year adjusted age. Pediatrics 1999;104(1 Pt 1):15‐21. - PubMed
Ohlsson 1992
-
- Ohlsson A, Calvert SA, Hosking M, Shennan AT. Randomized controlled trial of dexamethasone treatment in very‐low‐birth‐weight infants with ventilatory dependent chronic lung disease. Acta Paediatrica 1992;81(10):751‐6. - PubMed
Onland 2017a
Onland 2017b
Papile 1978
-
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 grams. Journal of Pediatrics 1978;92(4):529‐34. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2017
Saarela 1999
-
- Saarela T, Vaarala A, Lanning P, Koivisto M. Incidence, ultrasonic patterns and resolution of nephrocalcinosis in very low birth‐weight infants. Acta Paediatrica 1999;86(6):655‐60. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A (eds), GRADE Working Group. GRADE Handbook 2013. gdt.gradepro.org/app/handbook/handbook.html (accessed prior to 8 September 2017).
Shah 2001
-
- Shah V, Ohlsson A. Postnatal dexamethasone in the prevention of chronic lung disease. In: David TJ editor(s). Recent Advances in Paediatrics. London, England: Churchill Livingstone, 2001:77‐96.
Shah 2012
Shah 2017
Shinwell 2000
Shinwell 2016
-
- Shinwell ES, Portnov I, Meerpohl JJ, Karen T, Bassler D. Inhaled corticosteroids for bronchopulmonary dysplasia: A meta‐analysis. Pediatrics 2016;138(6):e20162511. - PubMed
Sinkin 1990
-
- Sinkin RA, Cox C, Phelps DL. Predicting risk for bronchopulmonary dysplasia: selection criteria for clinical trials. Pediatrics 1990;86(5):728‐36. - PubMed
Soll 1998
Stark 2001
-
- Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran EF, et al. Adverse effects of early dexamethasone treatment in extremely‐low‐birth‐weight infants. National Institute of Child Health and Human Development Neonatal Research Network. New England Journal of Medicine 2001;344(2):95‐101. - PubMed
Ward 2003
Watterberg 2010
-
- Watterberg KL, American Academy of Pediatrics. Committee of Fetus and Newborn. Policy statement ‐ postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics 2010;126(4):800‐8. - PubMed
Weichsel 1977
-
- Weichsel ME Jr. The therapeutic use of glucocorticoid hormones in the perinatal period: potential neurologic hazards. Annals of Neurology 1977;2(5):364‐6. - PubMed
Yeh 1998
-
- Yeh TF, Lin YJ, Huang CC, Chen YJ, Lin CH, Lin HC, et al. Early dexamethasone therapy in preterm infants: a follow up study. Pediatrics 1998;101(5):E7. - PubMed
Zubrow 1995
-
- Zubrow A, Hulman S, Kushner H, Falkner B. Determinants of blood pressure in infants admitted to neonatal intensive care units: a prospective multicenter study. Philadelphia Neonatal Blood Pressure Study Group. Journal of Perinatology 1995;15(6):470‐9. - PubMed
References to other published versions of this review
Shah 2003b
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

