Personalized medicine with biologics for severe type 2 asthma: current status and future prospects
- PMID: 29035619
- PMCID: PMC5800381
- DOI: 10.1080/19420862.2017.1392425
Personalized medicine with biologics for severe type 2 asthma: current status and future prospects
Abstract
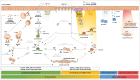
Asthma affects more than 300 million people worldwide and poses a large socioeconomic burden, particularly in the 5% to 10% of severe asthmatics. So far, each entry of new biologics in clinical trials has led to high expectations for treating all severe asthma forms, but the outcome has only been successful if the biologic, as add-on treatment, targeted specific patient subgroups. Indeed, we now realize that asthma is a heterogeneous disease with multiple phenotypes, based on distinct pathophysiological mechanisms, called endotypes. Thus, asthma therapy is gradually moving to a personalized medicine approach, tailored to individual's asthma endotypes identified through biomarkers. Here, we review the clinical efficacy of antibody-related therapeutics undergoing clinical trials, or those already approved, for the treatment of severe type 2 asthma. Biologics targeting type 2 cytokines have shown consistent efficacy, especially in patients with evidence of type 2 inflammation, suggesting that the future of asthma biologics is promising.
Keywords: asthma; biologic; biomarker; cytokine; personalized medicine.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
