Color opponency: tutorial
- PMID: 29036118
- PMCID: PMC6022826
- DOI: 10.1364/JOSAA.34.001099
Color opponency: tutorial
Abstract
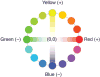
In dialogue, two color scientists introduce the topic of color opponency, as seen from the viewpoints of color appearance (psychophysics) and measurement of nerve cell responses (physiology). Points of difference as well as points of convergence between these viewpoints are explained. Key experiments from the psychophysical and physiological literature are covered in detail to help readers from these two broad fields understand each other's work.
Figures

References
-
- Hering E. Zur Lehre vom Lichtsinn. Gerald u. Söhne; 1878. Grundzüge einer Theorie des Farbensinnes (originally published in 1874) pp. 107–141.
-
- Turner RS. Vision studies in Germany: Helmholtz versus Hering. Osiris. 1993;8:80–103. - PubMed
-
- Young T. The Bakerian lecture: on the theory of light and colours. Philos. Trans. R. Soc. London. 1802;92:12–48.
-
- Mollon JD. The origins of modern color science. In: Shevell SK, editor. The Science of Color. Optical Society of America/Elsevier; 2003. pp. 1–39.
-
- Helmholtz H. In: Helmholtz’s Treatise on Physiological Optics. Southall JPC, editor. 1–2 Dover: 1962.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

