Progranulin-mediated deficiency of cathepsin D results in FTD and NCL-like phenotypes in neurons derived from FTD patients
- PMID: 29036611
- PMCID: PMC5886207
- DOI: 10.1093/hmg/ddx364
Progranulin-mediated deficiency of cathepsin D results in FTD and NCL-like phenotypes in neurons derived from FTD patients
Abstract
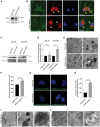
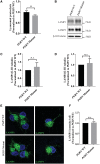
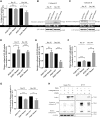
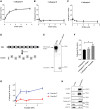
Frontotemporal dementia (FTD) encompasses a group of neurodegenerative disorders characterized by cognitive and behavioral impairments. Heterozygous mutations in progranulin (PGRN) cause familial FTD and result in decreased PGRN expression, while homozygous mutations result in complete loss of PGRN expression and lead to the neurodegenerative lysosomal storage disorder neuronal ceroid lipofuscinosis (NCL). However, how dose-dependent PGRN mutations contribute to these two different diseases is not well understood. Using iPSC-derived human cortical neurons from FTD patients harboring PGRN mutations, we demonstrate that PGRN mutant neurons exhibit decreased nuclear TDP-43 and increased insoluble TDP-43, as well as enlarged electron-dense vesicles, lipofuscin accumulation, fingerprint-like profiles and granular osmiophilic deposits, suggesting that both FTD and NCL-like pathology are present in PGRN patient neurons as compared to isogenic controls. PGRN mutant neurons also show impaired lysosomal proteolysis and decreased activity of the lysosomal enzyme cathepsin D. Furthermore, we find that PGRN interacts with cathepsin D, and that PGRN increases the activity of cathepsin D but not cathepsins B or L. Finally, we show that granulin E, a cleavage product of PGRN, is sufficient to increase cathepsin D activity. This functional relationship between PGRN and cathepsin D provides a possible explanation for overlapping NCL-like pathology observed in patients with mutations in PGRN or CTSD, the gene encoding cathepsin D. Together, our work identifies PGRN as an activator of lysosomal cathepsin D activity, and suggests that decreased cathepsin D activity due to loss of PGRN contributes to both FTD and NCL pathology in a dose-dependent manner.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

